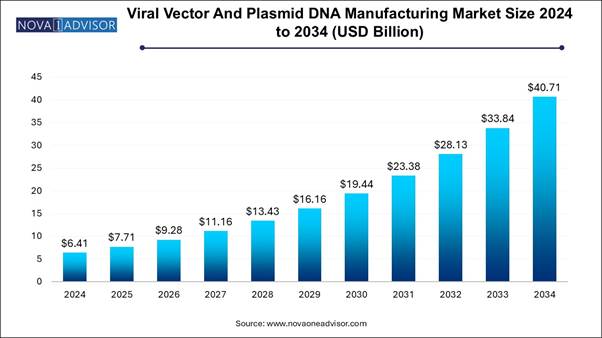
The global viral vector & plasmid DNA manufacturing market size is expected to be worth around 40.71 billion by 2034, increasing from USD 7.71 billion in 2025, representing a healthy CAGR of 20.3% from 2025 to 2034.
The viral vector & plasmid DNA manufacturing market is growing as increasing demand for cell and gene-based products for the treatment of complex diseases, which are considered impossible to treat, ultimately increases the manufacturing of viral vector and plasmid DNA. This manufacturing is essential for making the vital carriers that transport therapeutic genes. Viral vectors are widely applied in sectors such as gene therapy, creating an optimized manufacturing process important in the pharmaceutical industry. It directly targets the cause of a disease and changes the way a cell functions.
The Complete Study is Now Available for Immediate Access | Download the Sample Pages of this Report@ https://www.novaoneadvisor.com/report/sample/8613
Viral Vector & Plasmid DNA Manufacturing Market Highlights:
🔹The Adeno-associated virus (AAV) segment held the highest market share in 2024.
🔹The downstream processing segment held the highest market share in 2024.
🔹The vaccinology segment held the highest market share in 2024.
🔹The research institutes segment held the highest market share in 2024.
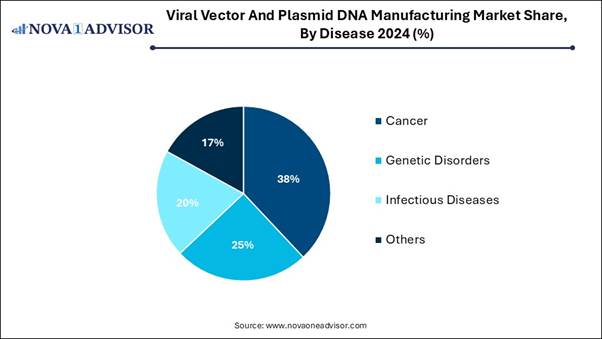
🔹The cancer segment held the highest market share in 2024.
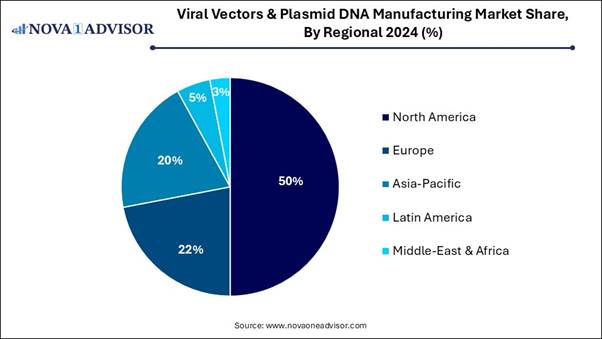
🔹North America dominated the global market with a share in 2024.
Market Overview and Industry Potential
Plasmid DNA plays a significant role in viral vector manufacturing, regulatory considerations, recent developments, and supply chain implications. Recent progressions have significantly enhanced the delivery efficacy and safety of viral vectors, directing different diseases like cardiovascular, cancer, and metabolic diseases. Vigorous government alignment and efficient supply chain management confirm the availability of advanced-quality plasmid DNA, eventually increasing the efficacy and safety of gene therapy applications.
The biotechnology landscape is experiencing a profound alteration, with plasmid DNA taking centre stage in vaccine development, gene therapy, and synthetic biology. With the increasing demand for high-quality plasmids due to advancements in cell and gene therapy, the industry is implementing ground-breaking manufacturing technologies.
Top Applications of Viral Vectors:
|
Application |
Examples |
|
Gene Therapy |
• Spinal Muscular Atrophy • Retinal Dystrophy • Metachromatic Leukodystrophy • Cancer therapies |
|
Vaccines |
· Ebola vaccine · Japanese encephalitis virus vaccine |
|
Protein Expression Systems |
• Targeting viral surface proteins • Production in plants for edible vaccines |
|
Immunotherapies |
• CAR-T cell therapies for blood cancers |
|
Gene Silencing |
• Delivering microRNAs to inhibit cancer cell growth |
Latest Trends of the Market
🔹In August 2025, ProBio, a leading contract development and manufacturing organization (CDMO) specializing in gene and cell therapy, announced the launch of its cGMP Adeno-Associated Virus (AAV) manufacturing services at its 128,000 sq. ft. state-of-the-art facility in Hopewell, New Jersey. This strategic expansion is designed to meet growing industry demand for high-quality viral vector production and reflects ProBio's ongoing commitment to supporting the advancement of life-changing gene therapies.
🔹In May 2025, Wacker Biotech and Expression Manufacturing announced a strategic partnership to accelerate development of lentiviral-based gene and cell therapies. Collaboration to offer comprehensive lentiviral vector development services from gene to plasmids to viral vector manufacturing
Recent Advancements in New Approach Methodologies: Market’s Largest Potential
Viral vector manufacturing is the most innovative advancement in gene and cell therapy, with the strength to transform medicinal treatments and improve people’s lives. Growing adoption of single-use machineries along with suspension-based cell values for the manufacturing of viral vectors. These technological advances need to be incorporated by the industry to advance the viral vector manufacturing industry. The adoption of suspension-based cell cultures results in a more easy and accessible process, which effortlessly leverages presenting single-use stimulated tank bioreactors that are utilized and optimized by the pharmaceutical industry.
Buy Now Full Report: https://www.novaoneadvisor.com/report/checkout/8613
Report Scope of Viral Vector And Plasmid DNA Manufacturing Market
|
Report Coverage |
Details |
|
Market Size in 2025 |
USD 7.71 Billion |
|
Market Size by 2034 |
USD 40.71 Billion |
|
Growth Rate From 2025 to 2034 |
CAGR of 20.3% |
|
Base Year |
2024 |
|
Forecast Period |
2025-2034 |
|
Segments Covered |
Vector Type, Workflow, Application, End-use, Disease, Region |
|
Market Analysis (Terms Used) |
Value (US$ Million/Billion) or (Volume/Units) |
|
Regional scope |
North America; Europe; Asia Pacific; Latin America; MEA |
|
Key Companies Profiled |
Merck KGaA, Lonza; FUJIFILM Diosynth Biotechnologies; Thermo Fisher Scientific; Cobra Biologics; Catalent Inc.; Wuxi Biologics; TakarBio Inc.; Waisman Biomanufacturing; Genezen laboratories; Batavia Biosciences; Miltenyi Biotec GmbH; SIRION Biotech GmbH; Virovek Incorporation; BioNTech IMFS GmbH; Audentes Therapeutics; BioMarin Pharmaceutical; RegenxBio, Inc. |
U.S. Viral Vector and Plasmid DNA Manufacturing Market Size and Growth 2025 to 2034
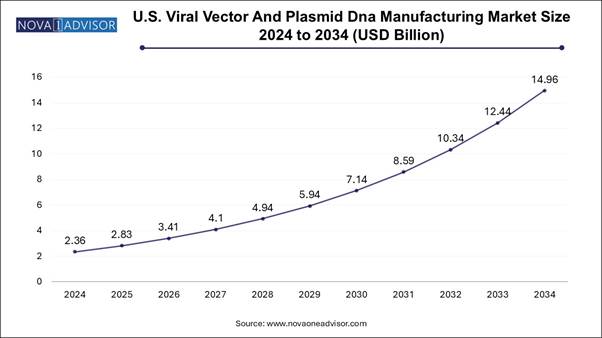
The U.S. viral vector and plasmid DNA manufacturing market size is evaluated at USD 2.36 billion in 2024 and is projected to be worth around USD 14.96 billion by 2034, growing at a CAGR of 18.27% from 2025 to 2034.
Regional Insight
North America led the viral vector and plasmid DNA manufacturing market in 2024, due to the strong presence of biotech and pharmaceutical organizations such as Amgen, Moderna, Johnson & Johnson, and Pfizer. Increasing investment in biomedical research and development from private and government sectors, such as NIH, drives the growth of the market.
🔹For Instance, In May 2024, Charles River Laboratories International, Inc. announced the introduction of its Modular and Fast Track viral vector technology (tech) transfer frameworks. Based on decades of viral vector contract development and manufacturing organization (CDMO) experience, the Company has designed a methodical program to drive successful, accelerated process transfer to its Maryland-based viral vector center of excellence (CoE) in as little as nine months.
In the U.S., the presence of advanced healthcare infrastructure for clinical trials supports rapid testing. The increasing prevalence of chronic diseases such as cancer, genetic disorders, and rare diseases increases the demand for viral vector and plasmid DNA manufacturing services.
Viral Vector & Plasmid DNA Manufacturing Market Segmentation Analysis:
By Vector Analysis:
The adeno-associated virus segment dominates in the viral vector & plasmid DNA manufacturing market, as it delivers the therapeutic gene to the central nervous system directly and offers long-term and purposeful correction of mutated or missing genes. The advancement of various serotypes and genetic targets for diverse cells assists in the treatment of neurodegenerative diseases stemming from different causes.
By Workflow Analysis:
The downstream manufacturing segment is dominating the market in 2024, as these downstream technologies make it possible to lower viral vector purification times from hours to minutes, also enhancing recovery. They allow scale-up, lower the process footprint, and enable more efficient facility operation. The downstream processes of viral vector manufacturing play a significant role in gene therapy. After the extension cell culture, the viral vector purification procedure begins, which includes eliminating contaminants and impurities to get a pure and effective viral vector.
By Application Analysis:
The research applications segment dominated the market in 2024, as plasmid DNA and viral vectors play a significant role in advanced biotechnology and biopharmaceuticals. From gene therapy to vaccine advancement, pDNA is a significant element driving developments in medicine. Confirming high-quality pDNA industrial is significant for attaining reliable and consistent results in different applications. pDNA is broadly used in synthetic biology and research, allowing progressions in genetic circuit design, drug discovery, and metabolic engineering.
By End User:
The research institutes segment dominated the market in 2024, as viral vector and plasmid DNA play a significant role in preclinical and clinical trials, with the support of Luxturna for a genetic retinal disease and CAR-T treatments for blood cancers. Viral vectors have developed essential tools in progressive therapeutics for a range of challenging medical conditions, like monogenic diseases and cardiac disorders.
By Disease Analysis:
The cancer segment dominated the market in 2024, as plasmid DNA introduces genes that inhibit tumor growth or encourage apoptosis in cancerous cells. Viral vectors are used to carry genes that change cancer cells back to normal cells or to replace a gene that isn’t working properly with a working copy of the gene. In cancer vaccines, viral vectors can be used to deliver precise cancer-related antigens in the body. The antigens stimulate the immune system to identify and kill the cancer cells.
Some of the prominent players in the viral vector and plasmid DNA manufacturing market include:
• Merck KGaA
• Lonza
• FUJIFILM Diosynth Biotechnologies
• Thermo Fisher Scientific
• Cobra Biologics
• Catalent Inc.
• Wuxi Biologics
• Takara Bio Inc.
• Waisman Biomanufacturing
• Genezen laboratories
• Batavia Biosciences
• Miltenyi Biotec GmbH
• SIRION Biotech GmbH
• Virovek Incorporation
• BioNTech IMFS GmbH
• Audentes Therapeutics
• BioMarin Pharmaceutical
• RegenxBio, Inc.
What is Going Around the Globe?
⬥︎ In June 2025, ProBio Inc., a global contract development and manufacturing organization (CDMO) specializing in cell and gene therapy, announced the opening of its flagship Cell and Gene Therapy Center of Excellence at the Princeton West Innovation Campus in Hopewell, New Jersey. The 128,000 sq ft GMP facility is purpose-built for manufacturing high-quality plasmid DNA and viral vectors, including AAV and lentiviral platforms, reflecting ProBio’s dedication to accelerating the delivery of transformative medicines
⬥︎ In July 2025, Addgene announced a strategic partnership with Promega to expand Addgene’s vast plasmid repository with the launch of the Promega Plasmid Collection. This new offering provides industry and academic researchers with broad access to reliable, high-value plasmids designed for studying target engagement, protein-protein interactions, and targeted protein degradation, unlocking powerful insights critical to drug discovery and other biological breakthroughs.
You can place an order or ask any questions, please feel free to contact at sales@novaoneadvisor.com | +1 804 441 9344
Segments Covered in the Report
This report forecasts revenue growth at country levels and provides an analysis of the latest industry trends in each of the sub-segments from 2021 to 2034. For this study, Nova one advisor, Inc. has segmented the viral vector and plasmid DNA manufacturing market
By Vector Type
• Adenovirus
• Retrovirus
• Adeno-Associated Virus (AAV)
• Lentivirus
• Plasmids
• Others
By Workflow
• Upstream Manufacturing
o Vector Amplification & Expansion
o Vector Recovery/Harvesting
• Downstream Manufacturing
o Purification
o Fill Finish
By Application
• Antisense & RNAi Therapy
• Gene Therapy
• Cell Therapy
• Vaccinology
• Research Applications
By End-use
• Pharmaceutical and Biopharmaceutical Companies
• Research Institutes
By Disease
• Cancer
• Genetic Disorders
• Infectious Diseases
• Others
By Regional
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East and Africa (MEA)
Immediate Delivery Available | Buy This Premium Research https://www.novaoneadvisor.com/report/checkout/8613
About-Us
Nova One Advisor is a global leader in market intelligence and strategic consulting, committed to delivering deep, data-driven insights that power innovation and transformation across industries. With a sharp focus on the evolving landscape of life sciences, we specialize in navigating the complexities of cell and gene therapy, drug development, and the oncology market, enabling our clients to lead in some of the most revolutionary and high-impact areas of healthcare.
Our expertise spans the entire biotech and pharmaceutical value chain, empowering startups, global enterprises, investors, and research institutions that are pioneering the next generation of therapies in regenerative medicine, oncology, and precision medicine.
Web: https://www.novaoneadvisor.com/
Contact Us
USA: +1 804 420 9370
Email: sales@novaoneadvisor.com
For Latest Update Follow Us: LinkedIn

