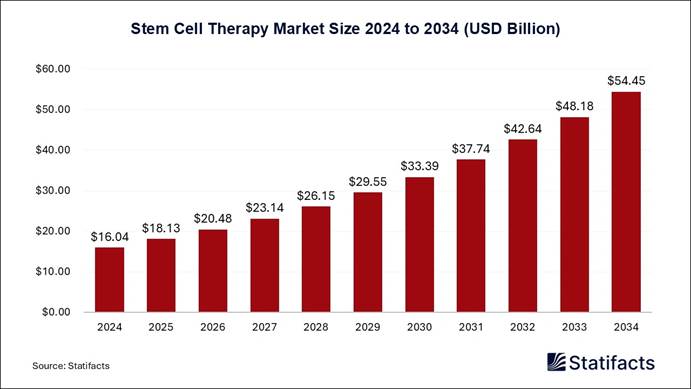
The global stem cell therapy market size is forecasted to reach around USD 54.45 billion by 2034, increasing from USD 16.04 billion in 2024 and representing a remarkable CAGR of 13% from 2025 to 2034.
In terms of revenue, the worldwide stem cell therapy market size was valued at approximately USD 16.04 billion in 2024 and is estimated to hit around USD 37.74 billion by 2031. Rising research and development activities undertaken by companies for stem cell therapy, growing prevalence of chronic diseases, increasing awareness regarding the availability of stem cell therapy in developing countries, and rising government initiatives for stem cell therapies are contributing to the growth of the market.
This report is readily available for immediate delivery. We can review it with you in a meeting to ensure data reliability and quality for decision-making.
Try Before You Buy – Get the Sample Report@ https://www.statifacts.com/stats/databook-download/8156
Stem Cell Therapy Market Highlights
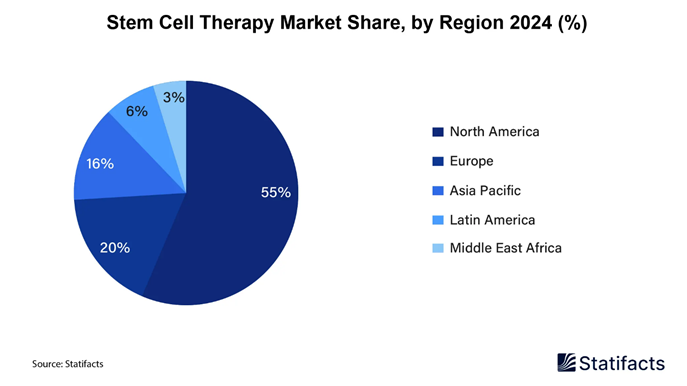
• North America dominated the global market share of 55% in 2024.
• Asia-Pacific is anticipated to grow at the fastest rate in the market during the forecast period.
• By product, the adult stem cells segment held a dominant presence in the market share of 86% in 2024.
• By product, the induced pluripotent stem cells (iPSCs) segment is expected to grow at the fastest rate in the market during the forecast period of 2025 to 2034.
• By therapy type, the allogenic stem cell therapy segment accounted for a considerable share of the market share 59.02% in 2024.
• By therapy type, the Autologous stem cell therapy segment is projected to experience the highest growth rate in the market between 2025 and 2034.
• By application, the regenerative medicine segment led the stem cell therapy market share of 93.02% in 2024.
• By application, the drug discovery and development segment is set to experience the fastest rate of market growth from 2025 to 2034.
• By technology, the cell acquisition segment maintained a leading position in the market in 2024.
• By technology, the cell production segment is predicted to witness significant growth in the market over the forecast period.
• By End User, the hospitals segment enjoyed a prominent position in the market during 2024.
• By End User, the research institutes segment will gain a significant share of the market over the studied period of 2025 to 2034.
What is the Remarkable Potential of the Stem Cell Therapy Market?
The stem cell therapy market refers to the production, distribution, and use of stem cell therapy, in which stem cells are cells with the potential to develop into many different types of cells in the body. They act as a repair system for the body. Stem cell therapy, also known as regenerative medicine, promotes the repair response of injured, dysfunctional, or diseased tissue using stem cells or their derivatives. Creating more effective therapeutic options worldwide, shortening approval timelines, accelerating the developing timeline, improving cryopreservation technologies, and automation in cryopreservation technologies are driving the growth of the stem cell therapy market.
Stem cell therapies can benefit people who have inherited metabolic conditions of metabolism, aplastic anemia, and immunodeficiencies. Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease. Stem cell benefits include reducing organ transplant rejections, addressing infertility, liver disease treatment, stem cells in eye health, diabetes treatment, treatment of autoimmune diseases, fighting cancer, tissue engineering, genetic disorders, reversing aging, understanding disease progression, drug development, neurological disease treatment, treatment of blood disorders, and regeneration of tissue.
Regulatory
Landscape and Challenges
As the
stem cell therapy market expands, navigating the complex regulatory landscape
remains a significant challenge. Regulatory agencies such as the U.S. FDA and
the European Medicines Agency (EMA) are increasingly recognizing the potential
of stem cell therapies, yet stringent regulatory hurdles persist.
Agencies are working on streamlining the approval processes for stem cell-based treatments, but issues like ethical concerns surrounding the use of embryonic stem cells and regulatory frameworks for induced pluripotent stem cells (iPSCs) still pose challenges. These hurdles have led to delays in approvals, despite the growing evidence supporting stem cell therapy's potential. However, ongoing efforts to establish clear regulations and accelerate approval timelines offer a promising outlook for the industry.
Case Study: Advancements in Parkinson’s Disease Treatment Using Stem Cells
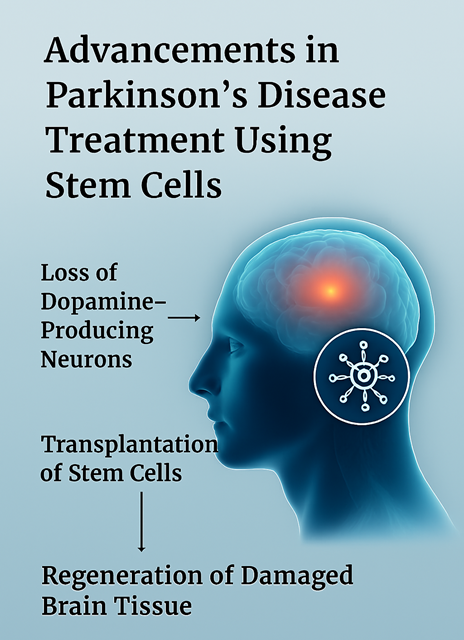
Recent breakthroughs in stem cell-based therapies for neurodegenerative diseases, particularly Parkinson's disease, have demonstrated the vast potential of regenerative medicine. Trials in both the USA and Japan are leading the charge, showing early signs of success in using stem cells to regenerate damaged brain tissue in patients with Parkinson's disease. These therapies focus on using stem cells to replace the lost dopamine-producing neurons in the brain, which are the primary cause of Parkinson's symptoms.
Why It's Relevant to Your Press Release:
This case study directly ties into the "regenerative medicine" segment mentioned in your press release, highlighting the power of stem cell therapy in treating chronic conditions that were previously considered untreatable. Parkinson's disease, being one of the most well-known neurological disorders, fits perfectly within the scope of stem cell applications as discussed in your market overview.
Key Highlights:
• Clinical Trials: In the USA, researchers are using stem cells derived from both adult and pluripotent sources to restore function in Parkinson’s patients. The trials focus on transplanting these cells into the brain to regenerate dopamine production.
• Treatment Outcomes: Initial results are promising, with patients showing improved motor functions and reduced symptoms. While it is still in the early stages, this marks a potential turning point in treating Parkinson’s disease, a condition with no current cure.
• Investment & Innovation: This area has seen significant investments, such as partnerships with major biotech firms and hospitals, facilitating further research and development in this field.
Engagement Strategy:
By presenting this case study, you highlight a key therapeutic application of stem cell therapy treating neurodegenerative diseases which is a rapidly growing area of interest in the market. This provides an opportunity to link the technological advancements and clinical successes outlined in your press release to tangible, real-world outcomes.
How to Leverage This Case Study for Engagement:
• Highlight Innovation: Emphasize how stem cell therapies are moving beyond simple regenerative solutions to address complex conditions like Parkinson's disease.
• Clinical Successes: Showcase the success stories and trials, demonstrating the real-world potential of stem cell therapies to engage readers who are interested in both medical advancements and patient outcomes.
• Industry Relevance: Tie the case study back to the market dynamics, such as the market’s growth projections, and demonstrate how treatments like this are likely to drive significant market expansion, aligning with your report's forecasts.
By focusing on Parkinson's disease as an application of stem cell therapy, you can engage both medical professionals and potential investors interested in the growing field of neurodegenerative treatment options. This case study not only adds credibility but also taps into the broader trend of applying regenerative medicine to complex health conditions.
Ready to Dive Deeper? Visit Here to Buy Databook and In-depth Report Now! https://www.statifacts.com/order-databook/8156
Cell Therapy Market Size 2024 to 2034
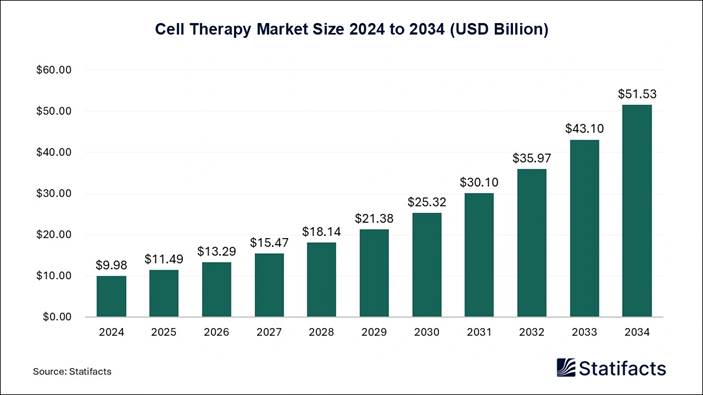
A related Statifacts report on the cell therapy market size highlights that the market was evaluated at USD 9.98 billion in 2024 and is anticipated to reach USD 51.53 billion by 2034, expanding at a CAGR of 17.84% from 2025 to 2034. The Cell Therapy Market uses living cells to treat diseases, including stem cell and immunotherapies. It’s growing fast, driven by advancements in biotechnology and applications in cancer, autoimmune diseases, and genetic disorders.
The Complete Study is Immediately Accessible | Download the Sample Pages of this Report@ https://www.statifacts.com/stats/databook-download/8501
Stem Cell Therapy Launched by Prominent Players
Clinical Successes:
Several clinical trials have shown significant progress with
stem cell-based therapies. For example, stem cell-derived retinal cells for
treating macular degeneration have demonstrated positive clinical outcomes,
with FDA approval anticipated in the near future. Additionally, stem cell
therapies are seeing success in the treatment of neurological diseases, with
breakthroughs in Parkinson's disease therapies being tested in clinical
settings.
|
Sr. No. |
Name of the Product |
Name of the Brand |
Product Specification |
|
1. |
Nanofiber-based cell culture system |
Cellevate |
The new system in the US and the US Food and Drug Administration has granted Regenerative Medicine Advanced Therapy (RMAT) designation to a cell replacement therapy for dry age-related molecular degeneration (dry AMD) and accepted a Biologics License Application for an in vivo gene therapy to treat Sanfilippo syndrome. |
|
2. |
Institute for Cell Therapy Discovery & Innovation |
The University of Texas MDAndersonn Cancer Center |
The new Institute for Cell Therapy Discovery & Innovation was launched to deliver transformational new therapies. |
|
3. |
India’s first-ever dedicated cell and gene therapy center of excellence (CoE) in Hyderabad |
Miltenyi Biotec |
With this launch, Miltenyi Biotec reinforces its commitment to India. It also furthers the vision of the company to advance cutting-edge biotechnology and faster innovation in India’s rapidly growing life sciences sector. |
|
4. |
Stem Cells Research Centers |
United Arab Emirates (UAE) University |
Stem Cells Research Centers was launched in support of a nationwide effort to advance cutting-edge healthcare and cement the country’s position as a global leader in regenerative medicine. |
Source: RegMedNet, MD Anderson, Bio Spectrum India, and The National News
Customize this Study as Per Your Requirement@ https://www.statifacts.com/stats/customization/8156
Major Trends in the Stem Cell Therapy Market
How do Technological Advancements Drive the Global Stem Cell Therapy Market?
Research and development activities for stem cell therapies and advancements in regenerative medicine drive the global stem cell therapy market.
• Research and development activities for Stem Cell Therapies: Researchers are studying stem cells to see if they can help to increase understanding of how diseases occur. By watching stem cells mature into cells in bones, heart muscle, nerves, and other organs and tissues, researchers may better understand how diseases and conditions develop. Additionally, stem cells can be used to create specialized tissues to test out drugs before prescribing them to humans. In the research and development activities, scientists are using stem cells to learn how the human body is made and why some cells develop abnormally and lead to problems like cancer and birth defects.
• Advancement in Regenerative Medicine: Recent advancements in regenerative medicine include the development of bioengineered organs and scaffolds that promote regeneration. 3D Bioprinting is an advanced technique that involves printing living cells in a layer-by-layer fashion to create three-dimensional tissue structures. The regenerative medicine future is bright with advancements promising to reshape the healthcare landscape. The possibilities are endless, from groundbreaking technologies like gene editing and 3D bioprinting to the pivotal role of stem cells.
Which Potential Factors Impose Significant Concerns Related to the Stem Cell Therapy Market’s Growth?
Ethical issues associated with stem cell therapy and regulatory hurdles are the potential factors that impose significant concerns related market’s growth.
• Ethical issues associated with stem cell therapy: Clinical applications of stem cell-based therapies can raise many ethical and safety concerns. Current ethical controversies regarding stem cell-based therapy are focused on the unlimited differentiation potential of iPSCs, which can be used in human cloning, as a risk for the generation of human and human-animal chimeras. Ethical challenges in stem cell therapy include ensuring broad availability of matches for those in need, determining access for both research and therapy, refining consent forms and processes, and protecting confidentiality in labeling and information linkage.
• Regulatory hurdles: Stem cell therapy regulations are the laws, rules, and policy governance concerning the sources, research, and uses in the treatment of stem cells in humans. FDA considers stem cell-based products to be somatic cellular therapies that do not warrant a distinct regulatory approach. Adult stem cells may not be able to be manipulated to produce all cell types, which limits how stem cells can be used to treat diseases. Adult stem cells are also more likely to contain irregularities due to environmental hazards like toxins or errors acquired by the cells during replication.
How Big Is the Development of the Stem Cell Therapy Platforms?
Stem cell therapy platform development offers several benefits. It is widely hoped that transplantation of stem cells will provide effective therapy for stroke, spinal cord injury, amyotrophic lateral sclerosis, Huntington’s Disease, Alzheimer’s disease, and Parkinson’s disease. These platforms help generate healthy cells to replace cells affected by disease. Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease.
Stem cell preservation enables us to use our body’s stem cells to repair and regenerate our body during illness. Stem cells can treat many diseases, repair organs, and rejuvenate tissue damaged by disease. Their use in diabetes, heart disease, and Alzheimer’s disease.
How Big is the Success of the North American Stem Cell Therapy Market?
North America dominated the global stem cell therapy market in 2024. Investment in infrastructure development, public-private partnerships, technological innovations and startups, high-growth regions, urbanization, income growth, regulatory & policy support, infrastructure environment, and consumer demand side drivers contribute to the growth of the market in the North American region.
• In February 2025, to launch the World’s first hospital-based autologous induced pluripotent stem cell (iPSC) Foundry, Cellino announced its collaboration with Mass General Brigham’s Gene and Cell Therapy Institute (GCTI). Powered by Cellino’s AI-driven Nebula Technology, this initiative sets the stage for a nationwide network of decentralized biomanufacturing hubs designed to deliver personalized cell and tissue therapies directly at the point of care.
How Is the Opportunistic Rise of Asia Pacific in the Stem Cell Therapy Market?
Asia Pacific is anticipated to grow at the fastest rate in the market during the forecast period. Rising R&D activities undertaken by companies for stem cell therapy, growing prevalence of chronic diseases, and increasing awareness of the availability of stem cell therapy in developing countries are driving the growth of the stem cell therapy market in the Asia Pacific region.
How Prestigious Is the Stem Cell Therapy Market in China, India, and Japan?
• In September 2024, to deliver the off-the-shelf induced pluripotent stem cell (iPSC)-based medicines, GC Therapeutics (GCTx) has propelled itself into the cell therapy space with $65m. GCTx’s “play and plug” platform TFome, developed in the lab of Professor George Church at Harvard Medical School, transforms iPSCs into cell type using a cell type using a comprehensive collection of human transcription factors.
• In May 2025, Japan’s first next-generation commercial production site for CAR-T cell therapies was established by Cellares, in collaboration with Mitsui Fudosan, a Japanese real estate developer. This Integrated Development and Manufacturing Organization (IDMO) Smart Factory is expected to provide employment to 350 people while enabling scalable and cost-effective cell therapy manufacturing, addressing an urgent need for patients in Japan and neighboring regions, according to Fabian Gerlinghaus, CEO and co-founder of Cellares.
Ready to Dive Deeper? Visit Here to Buy Databook and In-depth Report Now! https://www.statifacts.com/order-databook/8156
Stem Cell Therapy Market Scope
|
Report Attributes |
Statistics |
|
Market Size in 2024 |
USD 16.04 Billion |
|
Market Size in 2025 |
USD 18.13 Billion |
|
Market Size in 2030 |
USD 33.39 Billion |
|
Market Size in 2032 |
USD 42.64 Billion |
|
Market Size by 2034 |
USD 54.45 Billion |
|
CAGR 2025-2034 |
13% |
|
Leading Region in 2024 |
North America |
|
Fastest Growing Region |
Asia Pacific |
|
Base Year |
2024 |
|
Forecast Years |
2025-2034 |
|
Segments Covered |
Product, Therapy Type, Application, Technology, End User, and Region |
|
Region Covered |
North America, Europe, Asia Pacific, Latin America, Middle East & Africa (MEA) |
|
Key Players |
Caladrius, CELGENE CORPORATION, ReNeuron Group plc, Virgin Health Bank, Opexa Therapeutics, Inc, Pluristem Therapeutics Inc, STEMCELL Technologies Inc, Biovault family, Precious Cells International Ltd, Mesoblast Ltd, Seneca Biopharmaceuticals, Inc, and Others. |
Become a valued research partner with us - https://www.statifacts.com/schedule-meeting
Stem Cell Therapy Market Segmentation Analysis
Product Analysis
The adult stem cells segment underwent notable growth in the stem cell therapy market in 2024. Adult stem cells generate healthy cells to replace cells affected by disease. Stem cells can be guided into becoming specific cells that can be used in people to regenerate and repair tissues that have been damaged or affected by disease.
The induced pluripotent stem cells (iPSCs) segment will gain a significant share of the market over the studied period of 2025 to 2034. Due to the intrinsic properties of self-renewal and potential to differentiate into nearly any cell type in the body, patient-specific iPSCs could provide large quantities of disease-relevant cells and a variety of different cell types that were previously inaccessible, like neurons and cardiomyocytes.
Therapy Type Analysis
The allogenic stem cell therapy segment enjoyed a prominent position in the stem cell therapy market in 2024. Allogenic stem cell therapies can provide superior scalability to autologous treatments, increasing accessibility and potentially lowering manufacturing and patient costs. Allogenic stem cell therapy applications include regenerative medicine, solid organ transplantation, and hematological disorders.
The autologous stem cell therapy segment is predicted to witness significant growth in the market over the forecast period. One of the most exciting applications of autologous stem cell therapy is in cancer treatment. CAR T cell therapy has shown remarkable success in clinical trials. Autologous stem cell therapy is also used to treat orthopedic injuries, neurodegenerative diseases, and cardiovascular disease.
Application Analysis
The regenerative medicine segment captured a significant portion of the stem cell therapy market in 2024. As compared to traditional reactive medicine, which only repairs damage, regenerative medicines actually improve the health of the tissues and bone that it treats. It shows potential in treating cardiovascular diseases, neurological disorders, autoimmune diseases, and even specific types of cancer.
The Drug discovery and development segment is projected to expand rapidly in the market in the coming years. Drug discovery plays an important role in developing new therapies for the treatment of diseases that have previously been difficult to treat or have no effective treatment options. This leads to reduced healthcare costs, better healthcare outcomes, and enhanced quality of life.
Technology Analysis
The Cell acquisition segment maintained a leading position in the stem cell therapy market in 2024. Cell acquisition technology is increasingly used for more than voice calls. Improved handsets and the network’s increased data transfer speeds. Modern mobile phone networks use cells due to radio frequencies are limited, a shared resource. Handsets and cell sites change frequency under computer control and use low-power transmitters so that the usually limited number of radio frequencies can be simultaneously used by many callers with less interference.
The Cell production segment is anticipated to grow with the highest CAGR in the market during the studied years. Cell production benefits include improved production environment and quality control, improved capacity to produce high-volume, high-variety products at a fast pace. It also includes benefits of developing a highly versatile and efficient workforce, smaller work in progress inventory, substantial reduction in manufacturing lead time and waste, substantial reduction in manufacturing lead time and waste, enhanced capacity to produce high-variety, high-volume products at a fast pace, and improved production environment and quality control.
End-user Analysis
The Hospitals segment dominated the stem cell therapy market. Hospitals are a convenient place who need treatment for their illness. It also plays an important role in the community. By offering all treatments in one facility, hospitals can improve resources and prioritize patient care. This leads to reduced waiting times, faster diagnosis, and prompt initiation of treatment.
The Research Institutes segment is set to experience the fastest rate of market growth from 2025 to 2034. The benefits of research institutes include a network with current and future industry leaders and changemakers, access to top-of-the-line facilities built to support university research, study and explore more specialized fields, and learn from faculty with innovative knowledge.
See More Related Reports:
• The US cell therapy market size was calculated at USD 4.24 billion in 2024 and is predicted to attain around USD 21.41 billion by 2034, expanding at a CAGR of 17.57% from 2025 to 2034.
• The global automated and closed cell therapy processing system market size surpassed USD 1,530 million in 2024 and is predicted to reach around USD 12,630 million by 2034, registering a CAGR of 23.5% from 2025 to 2034.
• The global autologous cell therapy market size was evaluated at USD 5,430 million in 2024 and is expected to grow around USD 40,020 million by 2034, registering a CAGR of 22.11% from 2025 to 2034.
• The global cell therapy raw materials market size was evaluated at USD 4,690 million in 2024 and is expected to grow around USD 24,970 million by 2034, registering a CAGR of 18.2% from 2025 to 2034.
• The global cell therapy technologies market size was estimated at USD 6,560 million in 2024 and is projected to be worth around USD 34,040 million by 2034, growing at a CAGR of 17.9% from 2025 to 2034.
• The global cell therapy manufacturing market size is predicted to gain around USD 15,753 million by 2034 from USD 4,139 million in 2024 with a CAGR of 14.3%.
Competitive Landscape in the Stem Cell Therapy Market

• Caladrius: Caladrius Biosciences Inc. is among the first new breed of immunotherapy companies with proven expertise and unique experience in cell process optimization.
• CELGENE CORPORATION: This is a leading biopharma company, ideally positioned to discover, develop, and deliver innovative medicines for patients fighting serious diseases.
• ReNeuron Group plc: ReNeuron Group plc researches, develops, and commercializes cell-based therapies in the United Kingdom.
• Virgin Health Bank: They collect, process, and store stem cells from the umbilical cord blood of newborn infants.
• Opexa Therapeutics, Inc: Opexa Therapeutics, Inc. is a publically traded biotechnology company developing personalized immunotherapies with the potential to treat major illnesses, including multiple sclerosis (MS) as well as other autoimmune diseases like neuromyelitis optica (NMO).
• Pluristem Therapeutics Inc: This is a bio-therapeutics company dedicated to the commercialization of unrelated donor patient (allogenic) cell therapy products.
• STEMCELL Technologies Inc: STEMCELL Technologies Inc. develops cell culture media, cell separation systems, instruments, and other reagents for use in life science research.
• Biovault family: Biovault Family offers private storage of umbilical cord blood and umbilical cord tissue.
• Precious Cells International Ltd: Precious Cells International Ltd. provides banking services for cord blood stem cells, cord tissue cells, and dental pulp cells.
• Mesoblast Ltd: Mesoblast Ltd is a world leader in developing allogenic (off-the-shelf) cellular medicines for the treatment of severe and life-threatening inflammatory conditions.
• Seneca Biopharmaceuticals, Inc.: This is a clinical-stage biopharmaceutical company focused on researching and developing new treatments for nervous system diseases of unmet medical need.
What is Around the Globe?
• In July 2025, the Ambitious Stem Cell Program to Restore Brain Function was launched by the US Department of Health and Human Services (HHS). The program will specifically target the largest region in the brain, the neocortex, which is responsible for higher cognitive functioning, including attention, thought, perception, and episodic memory.
• In November 2024, the upcoming launch of the newly developed Single Cell Cloning Platform XT at the Cell 2024 event in London (UK) was announced by iotaSciences. The Single-cell Cloning Platform XT is an instrument based on the existing Single-Cell Cloning Platform and iotaSciences’ proprietary fluid shaping technology.
Stem Cell Therapy Market Segments Covered in the Report
By Product
• Adult Stem Cells (ASCs)
o Hematopoietic
o Mesenchymal
o Neural
o Epithelial/Skin
o Others
• Human Embryonic Stem Cells (HESCs)
• Induced Pluripotent Stem Cells (iPSCs)
• Very Small Embryonic-Like Stem Cells
By Therapy Type
• Autologous
• Allogenic
By Application
• Regenerative Medicine
o Neurology
o Orthopedics
o Oncology
o Hematology
o Cardiovascular and Myocardial Infarction
o Injuries
o Diabetes
o Liver Disorder
o Incontinence
o Others
• Drug Discovery and Development
By Technology
• Cell Acquisition
Bone Marrow Harvest
Umbilical Blood Cord
Apheresis
• Cell Production
o Therapeutic Cloning
o In-vitro Fertilization
o Cell Culture
o Isolation
• Cryopreservation
• Expansion and Sub-Culture
By End User
• Hospitals
• Research institutes
• surgical institutes
• Orders
By Region
• North America
• Latin America
• Europe
• Asia-pacific
• Middle and East Africa
Ready to Dive Deeper? Visit Here to Buy Databook and In-depth Report Now! https://www.statifacts.com/stats/databook-download/6997
𝐀𝐛𝐨𝐮𝐭 𝐔𝐬:
Statifacts is a global market intelligence and consulting leader, committed to delivering deep strategic insights that fuel innovation and transformation. With a sharp focus on the fast-evolving landscape of life sciences, we excel at navigating the intricacies of cell and gene therapies, oncology, and drug development. We empower our clients, ranging from biotech pioneers to institutional investors with the intelligence needed to lead in high-impact areas like regenerative medicine, cancer therapeutics, and precision health. Our broad expertise across the pharma-biotech value chain is backed by robust, statistically driven data for every market we cover, ensuring decisions are informed, forward-looking, and built for impact.
Statifacts offers subscription services for data and analytics insights. This page provides options to explore and purchase a subscription tailored to your needs, granting access to valuable statistical resources and tools. Access here - https://www.statifacts.com/get-a-subscription
Connect with Us
Ballindamm 22, 20095 Hamburg, Germany
Europe: +44 7383092044
Web: https://www.statifacts.com/
For Latest Update Follow Us: https://www.linkedin.com/company/statifacts
Our Trusted Data Partners:
Precedence Research | Towards Healthcare | Nova One Advisor
FAQs
Q.
What is driving growth in the U.S. stem cell therapy sector?
Ans. The rising incidence of chronic and degenerative diseases, such as
blood disorders, neurological conditions, and orthopedic injuries, is
increasing reliance on innovative therapies. Strong funding support and an
expanding pipeline of clinical trials are accelerating development.
Q.
Which types of stem cells and therapy formats are most prominent in the U.S.?
Ans. Adult stem cells, particularly hematopoietic and mesenchymal
types, lead usage, while allogeneic (donor-based) therapies dominate. Induced
pluripotent stem cells (iPSCs) are rapidly gaining ground for their broader
therapeutic potential.
Q.
Which applications and end users are central to the market?
Ans. Regenerative medicine remains the primary application area, with
hospitals and cell therapy centers as key service providers. Drug discovery
using stem cells, especially iPSCs, is also growing as a secondary use case.
Q.
How are regulatory developments impacting access to treatments?
Ans. The FDA’s accelerated pathways, such as RMAT, orphan drug, and
fast-track designations, have enabled faster approvals for important therapies
like Ryoncil (remestemcel-L).
Q.
What are the biggest challenges and innovation opportunities in the U.S.
market?
Ans. High manufacturing costs, regulatory complexity, and technical
hurdles in cell processing limit scale-up. But advances in bioprocessing,
AI-driven design, cost control, and emerging uses in rare and inflammatory
diseases present major prospects.

