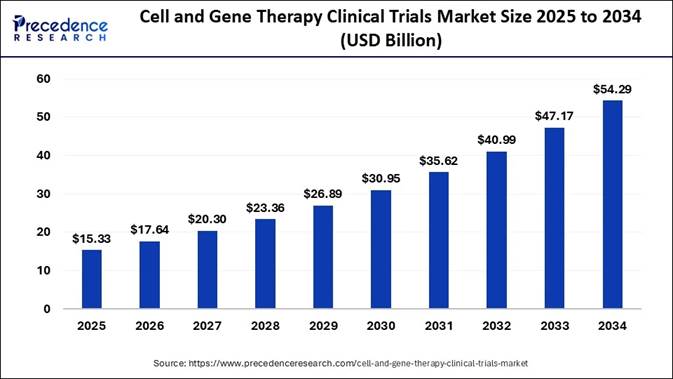
According to Precedence Research, the global cell and gene therapy clinical trials market size is calculated at USD 15.33 billion in 2025 and is expected to hit approximately USD 54.29 billion by 2034. The North America market size was estimated at USD 6.53 billion in 2024 and is expected to grow at a fastest CAGR of 15.19% during the forecast period.
In terms of CAGR, the cell and gene therapy clinical trials market is expected to expand at a healthy CAGR of 15.09% between 2024 and 2034. It is projected to rise from USD 15.33 billion in 2025 to USD 54.29 billion by 2034.
An increase in R&D financing, increasing patient demand for novel therapies, rising interest in cell and gene therapies for cancer treatment, and a supportive regulatory climate are the main market drivers. A brand-new front in the battle against numerous fatal illnesses, such as cancers and uncommon genetic conditions, is cell and gene therapy (CGT). It stands for the most recent round of invention in the life sciences sector.
Note: This report is readily
available for immediate delivery. We can review it with you in a meeting to
ensure data reliability and quality for decision-making.
📥 Download Sample Pages for
Informed Decision-Making 👇 https://www.precedenceresearch.com/sample/2881
Cell and Gene Therapy Clinical Trials Market - Key Takeaways
🔹In terms of revenue, the cell and gene therapy clinical trials market was valued at USD 13.32 billion in 2024.
🔹The market is projected to surpass USD 54.29 billion by 2034.
🔹It is expected to grow at a healthy CAGR of 15.09% from 2025 to 2034.
🔹North America accounted for the largest market share of 49% in 2024.
🔹Asia Pacific is expected to grow at the fastest CAGR from 2024 to 2034.
🔹By phase, the phase II segment held the major market share of 54% in 2024.
🔹By indication, the oncology segment contributed the highest market share of 47.70% in 2024.
Cell and Gene Therapy Clinical Trials Market Overview and Industry Potential
From Research to Reality: The Rise of Advanced Cell and Gene Therapies
The cell and gene therapy clinical trials market is expected to experience significant growth owing to the increased need for treating diseases at their root. Moreover, many major companies have been actively investing in investments for R&D activities for the development of advanced therapies in the past few years. Also, factors such ate fast fast-paced lifestyles and dietary changes are creating major diseases that can contribute to the growth of the market in the upcoming years.
➤ Get the Full Report @ https://www.precedenceresearch.com/cell-and-gene-therapy-clinical-trials-market
What is the Opportunity for Cell and Gene Therapy Clinical Trials Market?
Rising Demand for Scalable Solutions Fuels Supplier Growth
The increased use of advanced tools and the release of innovative tools are expected to create lucrative opportunities for manufacturers in the upcoming years. Moreover, as the major companies enter the industry, the need for reliable tools increases, where the suppliers can gain an immense industry share by supplying high-quality and scalable tools to the companies in the upcoming years.
What is the Major Challenge for Cell and Gene Therapy Clinical Trials Market?
Complex Compliance Requirements Threaten Future Market Growth
The increasingly complex and strict regulations are expected to hamper the industry's growth in the coming years. Moreover, several countries have different rules and regulations that can affect the Chinese growth through their implementation of these rules. Also, sometimes these changes are costly, where the new entrants and mid-sized businesses can face the growth barriers in the upcoming years, as per the future industry expectations.
The EU’s ATMP regulations and Japan’s PMDA guidelines require specialized documentation, increasing trial approval times by up to 12 months. This creates barriers for mid-sized biotech firms operating in multiple countries.
Also Read 👉 How the Cell Therapy Market Is Powering the Future of Regenerative Medicine
Key Trends in the Cell and Gene Therapy Clinical Trials Market
• Rapid Expansion of Oncology-Focused
Trials: Oncology remains the dominant
therapeutic area, with a high concentration of trials targeting hematological
malignancies (e.g., leukemia, lymphoma) and growing interest in solid tumors
using CAR-T, TIL, and TCR therapies.
• Acceleration of Rare
Disease Research: Cell
and gene therapies are increasingly being developed for rare genetic disorders
such as spinal muscular atrophy (SMA), Duchenne muscular dystrophy, and sickle
cell disease, areas with high unmet needs and regulatory incentives.
• Emergence of In Vivo Gene Editing
Technologies: CRISPR base gene editing, and prime editing
platforms are entering clinical trials for direct in vivo application, enabling
more precise and durable treatments with reduced need for ex vivo manipulation.
• Regulatory Fast-Tracking and Global Harmonization: Agencies like the
FDA (USA), EMA (Europe), and PMDA (Japan) are expanding fast-track programs
such as RMAT, Breakthrough Therapy, and Sakigake to support earlier trial
access and faster approvals, while efforts to align global trial protocols are
increasing.
• Rising Role of CDMOs in Trial Support: Specialized Cell and Gene Therapy CDMOs are playing a critical
role by offering GMP-compliant manufacturing, vector production, and clinical-scale
logistics, helping sponsors overcome technical and regulatory barriers.
This trend is accelerating as biotech firms outsource complex manufacturing to
CDMOs like Catalent, Lonza, WuXi Advanced Therapies, and OmniaBio.
Also Read 👉 Why the Pharmaceutical CDMO Market Is Reshaping Global Drug Manufacturing
Market Scope of Cell and Gene Therapy Clinical Trials|
Report Attributes |
Key Statistics |
|
Market Size in 2024 |
USD 13.32 Billion |
|
Market Size in 2025 |
USD 15.33 Billion |
|
Market Size by 2034 |
USD 54.29 Billion |
|
CAGR from 2025 to 2034 |
15.09% |
|
Leading Region in 2024 |
North America |
|
Fastest Growing Region |
Asia-Pacific |
|
Base Year |
2024 |
|
Forecast Period |
2025 to 2034 |
|
Segments Covered |
Phase, Indication, and Region |
|
Regions Covered |
North America, Europe, Asia-Pacific, Latin America and Middle East & Africa |
Become a valued research partner with us -https://www.precedenceresearch.com/schedule-meeting
Cell and Gene Therapy Clinical Trials Market Key Regional Analysis:
How Has a Strong Research Base Propelled North America’s Biotech Growth?
North America held the dominant share of the cell and gene therapy clinical trials market in 2024, owing to the presence of a well-established research industry and rapid adoption of medical technology across the region. Moreover, the region is gaining benefits from factors such as the presence of skilled professionals and easy regulatory access through the government agencies. Moreover, the presence of the major biotech companies is driving the growth of the market in the region nowadays.
💡 MORE FOR YOU:
➢ Cell and Gene Therapy Market Size Rapidly Approaching $117.46 Billion by 2034
➢ CAR T-Cell Therapy Market Size Worth USD 128.55 Billion by 2034
➢ Active Pharmaceutical Ingredients Market Set to Hit USD 405.09 Billion by 2034
➢ Cancer Therapeutics Market Size Expected to Hit USD 469.38 Bn by 2034
Country-level Analysis:
How Big is the U.S. Cell and Gene Therapy Clinical Trials Market?
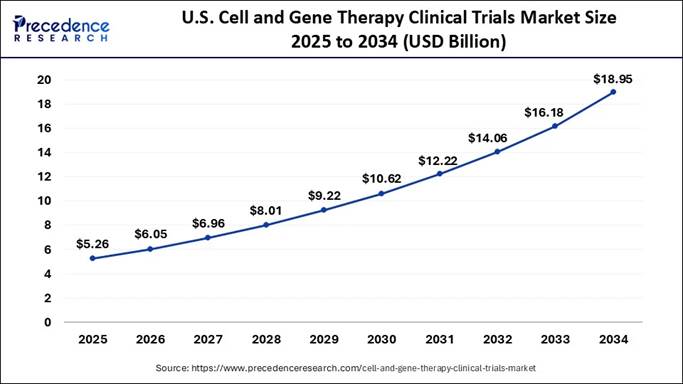
According to Precedence Research, the U.S. cell and gene therapy clinical trials market size reached USD 4.57 billion in 2024 and is expected to grow from USD 5.26 billion in 2025 to USD 18.95 billion by 2034, growing at a CAGR of 15.28% from 2025 to 2034.
The Full Study is Readily Available |
Download the Sample Pages of this Report @ https://www.precedenceresearch.com/sample/2881
United States: Leading Innovation in Gene Editing and CAR-T Trials
Academic institutions such as the University of Pennsylvania, MD Anderson Cancer Center, and the National Institutes of Health (NIH) play a pivotal role in trial development, while biotech companies like Vertex, CRISPR Therapeutics, and Bluebird Bio are advancing late-stage gene editing and CAR-T therapies. Clinical trial activity spans all phases, with a noticeable rise in early-stage (Phase I) trials for conditions like sickle cell anemia and inherited retinal diseases.
Canada: Emerging Hub for Early-Phase and Rare Disease Research
While smaller in scale compared to the U.S., Canada is positioning itself as a regional hub for early-phase trials and translational research. Institutions such as the University Health Network (UHN), BC Cancer, and the Ottawa Hospital Research Institute are at the forefront of clinical studies in oncology, autoimmune conditions, and rare inherited disorders. Health Canada has taken steps to streamline clinical trial approvals, especially for advanced therapies, which is encouraging cross-border collaborations and multinational studies.
Europe:
Countries like Germany and the UK are leading CGT trials, supported by centralized EMA approval pathways. The region accounts for over 25% of ongoing trials, driven by strong academic-industry collaborations.
Latin America:
Brazil and Mexico are emerging as attractive destinations for early-phase trials due to cost-effective infrastructure and growing clinical expertise.
Also Read 👉 Why CAR T-Cell Therapy Is Transforming the Future of Cancer Care
What’s Fuelling the Biotech Boom in the Asia Pacific Region?
Asia Pacific is expected to expand notably during the forecast period, owing to factors like low cost and increased investment in the biotechnology field in recent years. Moreover, the regional countries such as India, China, and South Korea are seen under the heavy development of the advanced research facilities in their region, as per the recent regional survey. Furthermore, the increased demand for precision medicine is expected to contribute heavily to the regional growth during the forecast period.
India and Japan Country-level Analysis:
➢ India: Homegrown CAR-T therapies are being developed and deployed. NexCAR19, developed by ImmunoACT/IIT Bombay, received approval in October 2023 and began providing treatment in India in 2024, with notable response rates in lymphoma/leukemia patients. Infrastructure and manufacturing are expanding: facilities in the country (ImmunoACT) and upcoming GMP-compliant capacity are under development. Government-backed projects and partnerships with institutions like Tata Memorial reinforce domestic scaling
➢ Japan: Notable developments include iPSC-based therapies, such as Kyoto University’s upcoming automated production of autologous iPS cells starting in April 2025, aimed at reducing manufacturing costs and enabling personalized treatment research. Japanese biotech companies are also pivoting towards CAR-T and CAR-NK therapies. For instance, Healios is advancing its iPSC-derived engineered NK cells (eNK) toward clinical trials for solid tumors, combining gene editing with immune checkpoint strategies
Also Read 👉 Engineered T Cells Market: Unlocking the Future of Precision Immunotherapy
Case Study: India’s First Commercial CAR-T Therapy – NexCAR19

In 2024, India approved its first indigenous CAR-T cell therapy, NexCAR19, developed by ImmunoACT in collaboration with IIT Bombay and Tata Memorial Hospital. This breakthrough represents a major milestone in Asia’s clinical trials landscape, especially for resource-efficient CGT development and delivery.
Challenge:
India previously lacked local manufacturing and delivery infrastructure for CAR-T therapies, resulting in limited patient access and dependency on imported treatments costing over $400,000 per dose.
Solution:
With support from the Department of Biotechnology, the ImmunoACT team developed an affordable, homegrown CAR-T therapy for B-cell leukemia and lymphoma. They launched clinical trials at Tata Memorial Hospital, achieving notable response rates and manageable toxicity in Phase I/II trials.
Results:
🔸 Regulatory Approval: NexCAR19 was approved in October 2023 under India's advanced therapy framework.
🔸 Commercial Launch: Initiated in Q1 2024 across select hospitals.
🔸 Cost Impact: Delivered at 1/10th the cost of international CAR-T therapies.
🔸 Trial Expansion: Phase II trials expanded to additional cancer centers by mid-2024.
Strategic Implications:
🔸 India emerged as a regional CGT clinical trials hub with cost-effective models.
🔸 Showcased the power of academic-industry-government collaboration.
🔸 Attracted interest from global biotech firms for cross-border trial partnerships.
“This development proves that with the right ecosystem, cutting-edge cell therapy can be made affordable and scalable in emerging markets,” said Dr. Rahul Purwar, Founder of ImmunoACT and Professor at IIT Bombay.
Where Is Cell & Gene Therapy Headed Next? Download the latest market report and explore what’s fueling biotech’s clinical trial boom. Get the Report@ https://www.precedenceresearch.com/checkout/2881
Cell and Gene Therapy Clinical Trials Market Segmentation Analysis:
By Phase Analysis:
Why Did the Phase II Segment Dominate the Cell and Gene Therapy Clinical Trials Market in 2024?
The phase II silencing segment held the largest share of the cell and gene therapy clinical trials market in 2024, owing to it being considered the crucial step in the clinical trial where this test both effectiveness of the treatment and safety in the larger group of patients. Moreover, several cell and gene therapies are seen under the phase II, which indicates how phase II has received this much attention in the current period.
On the other hand, the phase III segment is expected to grow at a notable rate, akin to this phase provides accurate information about the effectiveness of treatment of the large-scale patient group, which is bigger than phase II. Also, several major companies are actively seen in getting their medicine approvals after the phase III trials by the government agencies in recent years.
By Indication Analysis:
How the Oncology Segment Maintains Its Dominance in the Current Cell and Gene Clinical Trial Industry?
The oncology segment held the largest share of the market in 2024 because many cell and gene therapies are designed to treat cancer. These therapies, like CAR-T cell treatments, have shown strong results in targeting blood cancers and solid tumors. Cancer remains one of the most urgent and researched diseases, attracting major funding and trial activity. Clinical trials for cancer treatments are often prioritized due to high patient need and market demand.
The infectious diseases segment is expected to grow at a significant rate in the upcoming years, because of increasing global attention on developing advanced treatments for viruses and bacteria. After the COVID-19 pandemic, there is a major push to use gene-based tools to fight infectious threats more effectively. Gene therapy can be used to boost immunity or deliver targeted treatments for diseases like HIV, hepatitis, and emerging viruses.
Related Topics You May Find Useful:
➢ Cell and Gene Therapy Bioanalytical Testing Services Market: Meet rising demand as advanced testing accelerates CGT commercialization
➢ Cell and Gene Therapy Infrastructure and Delivery Models Market: Explore how scalable platforms are transforming next-gen therapy logistics
➢ Cell and Gene Therapy Patient Access and Reimbursement Market: Track how pricing models and payer strategies are shaping patient access
➢ Stem Cells Market: Uncover the growing role of stem cells in regenerative and precision medicine
➢ Automated and Closed Cell Therapy Market: See how automation is streamlining cell manufacturing and reducing variability
Key Companies & Market Share Insights
The key market players can expect profitable growth possibilities as research and development investments are rising at a rapid pace. In addition, the market players are implementing key marketing strategies for the growth and development of the cell and gene therapy clinical trials market. REGENXBIO Inc. and Ultragenyx Pharmaceutical Inc., for example, deployed new technology for the development of gene therapy treatments in March 2020.
Cell and Gene Therapy Clinical Trials Market Top Companies

The cell and gene therapy clinical trials market is dominated by key players who collectively command the largest market share and significantly influence industry trends.
→ IQVIA
→ ICON Plc
→ Laboratory Corporation of America Holdings
→ Charles River Laboratories International, Inc.
→ PAREXEL International Corp.
→ Syneos Health
→ Medpace Holdings, Inc.
→ PPD Inc.
→ Novotech
→ Veristat, LLC
What is Going Around the Globe?
🔸 In 2024, Bharat Biotech introduced the latest cell and gene therapy in Hyderabad and had a production facility in Genome Valley in Hyderabad, India. The production facility aims to produce major materials for anticancer and genetic disorders, which are high-titer viral vectors. (Source: https://www.moneycontrol.com)
🔸 In 2024, Novartis created a partnership with Voyager Therapeutics with a signed capsid license agreement. The motive of the collaboration is to develop and research gene therapies for Huntington's disease. Similarly, their expert team is expected to research the development of gene therapy for spinal muscular atrophy (SMA). (Source- https://ir.voyagertherapeutics.com)
🔸 In 2024, the first gene therapy was approved on 25 April by Pfizer, named Fidanacogene elaparvovec-dzkt (Beqvez). This treatment is mainly for adults with moderate to severe hemophilia B. This gene therapy is available for a limited patients who do not have antibodies to fight the virus called serotype Rh74var (AAVRh74var) capsid. (Source: https://www.isctglobal.org)
🔸 In 2025, the first cell therapy, CAR T, was unveiled in India. This cell therapy will be used in treating adult B-cell Non-Hodgkin Lymphoma, which is similar to blood cancer that affects the lymphatic system. (Source: https://www.newindianexpress.com)
Cell and Gene Therapy Clinical Trials Market Segmentation:
By Phase
• Phase I
• Phase II
• Phase III
• Phase IV
By Indication
• Oncology
• Cardiology
• CNS
• Musculoskeletal
• Infectious Diseases
• Dermatology
• Endocrine, Metabolic, Genetic
• Immunology & Inflammation
• Ophthalmology
• Hematology
• Gastroenterology
• Others
By Geography
• North America
• Europe
• Asia Pacific
• Latin America
• Middle East & Africa (MEA)
Thanks for reading you can also get individual chapter-wise sections or region-wise report versions such as North America, Europe, or Asia Pacific.
⏳ Don’t Miss Out! | ⚡ Instant Access to This Exclusive Report 👉 https://www.precedenceresearch.com/checkout/2881
You can place an order or ask any questions, please feel free to contact at sales@precedenceresearch.com | +1 804 441 9344
Stay Ahead with Precedence Research Subscriptions
Unlock exclusive access to powerful market intelligence, real-time data, and forward-looking insights, tailored to your business. From trend tracking to competitive analysis, our subscription plans keep you informed, agile, and ahead of the curve.
Browse Our Subscription Plans@ https://www.precedenceresearch.com/get-a-subscription
About Us
Precedence Research is a global market intelligence and consulting powerhouse, dedicated to unlocking deep strategic insights that drive innovation and transformation. With a laser focus on the dynamic world of life sciences, we specialize in decoding the complexities of cell and gene therapy, drug development, and oncology markets, helping our clients stay ahead in some of the most cutting-edge and high-stakes domains in healthcare. Our expertise spans across the biotech and pharmaceutical ecosystem, serving innovators, investors, and institutions that are redefining what’s possible in regenerative medicine, cancer care, precision therapeutics, and beyond.
Web: https://www.precedenceresearch.com
Our Trusted Data Partners:
Towards Healthcare | Statifacts | Nova One Advisor | Market Stats Insight
Get Recent News 👉 https://www.precedenceresearch.com/news
For Latest Update Follow Us:

