Neuroscience
South Korea-based Genuv Inc. announced the publication of a preclinical study showing the potential of Mekinist, approved by the FDA for melanoma, in Alzheimer’s disease.
Researchers recently developed a peptide called A1R-CT that could ultimately have therapeutic applications in both epilepsy and Alzheimer’s disease.
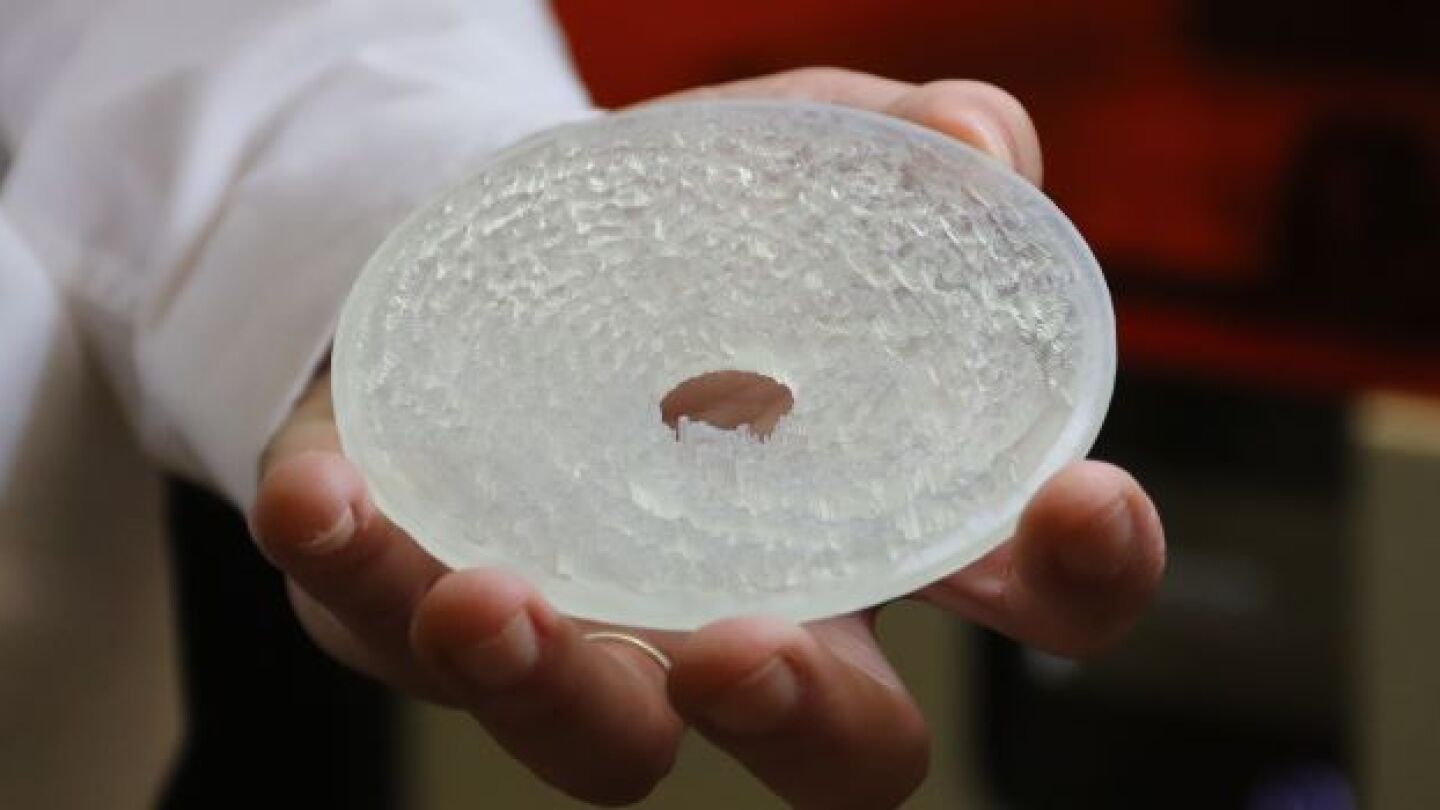
The 3D-printed acoustic hologram could be very important to the delivery of certain therapeutics for Alzheimer’s Disease (AD) and other neurological disorders.
A new study on Alzheimer’s disease ties the brain’s immune response to the condition, while scientists have identified a better antibody treatment for cancer and more research news.
Using Cerevance’s proprietary NNETSseq technology platform, Merck and Cerevance have partnered to identify new targets for the treatment of Alzheimer’s disease.
If the FDA ultimately votes to approve Eli Lilly’s donanemab or Eisai’s lecanemab – both anti-amyloid beta (Aβ) antibodies – what impact will it make on Alzheimer’s disease?
This week’s highlights in neurological diseases include Acadia receiving a CRL for its ADP drug, Sosei and Neurocrine’s schizophrenia drug study moving on to Phase II.
This week, drugs developed by Neurocrine Biosciences, Akebia, Eliem and Athira all failed to meet expectations in clinical trials.
Researchers have found that two common viruses—the varicella zoster and herpes simplex viruses—likely constitute a pathway that leads to Alzheimer’s disease.
Roche signed a big $6 billion-plus collaboration deal with Poseida on blood cancers while its partner, AC Immune, parsed failed data on an Alzheimer’s drug to suggest a positive spin.
PRESS RELEASES