A Research Collaboration Between LuminUltra, Dalhousie University and Halifax Water Demonstrates Wastewater Data Is a Powerful Tool in Early Detection and Tracking COVID-19 Prevalence within Communities
A Research Collaboration Between LuminUltra, Dalhousie University and Halifax Water Demonstrates Wastewater Data Is a Powerful Tool in Early Detection and Tracking COVID-19 Prevalence within Communities
FREDERICTON, NB, Oct. 21, 2020 /PRNewswire/ - LuminUltra, a Canada-based biotechnology leader and portfolio company of XPV Water Partners, recently filed a patent for the first complete, rapid, and on-site COVID-19 wastewater testing solution that will make non-invasive community health assessment far more accessible to both the public and private sector around the world.
This innovation builds upon LuminUltra's 20-plus years of leadership in the measurement of pathogens and microbes in wastewater systems, and is a direct result of a research collaboration between LuminUltra, Dalhousie University and Halifax Water. Scientists from LuminUltra and Dalhousie University collaborated to assess real-world wastewater samples provided by Halifax Water, in order to refine and improve the process needed to prepare a sample for accurate wastewater testing. Ultimately, the innovation in the RNA extraction and concentration process resulting from the team's research has eliminated the need for additional complicated equipment, greatly simplifying the process from sample collection to result while increasing accuracy and consistency of results.
"The idea of wastewater surveillance testing has been advocated by researchers around the world since the onset of the COVID-19 pandemic," said Pat Whalen, President and CEO of LuminUltra. "Until now, wastewater testing has been complicated, expensive and time consuming – meaning the potentially life-saving technique was reserved for niche subgroups under the watchful eye of researchers. We have been determined to make this surveillance tool more accessible to communities everywhere, allowing for a game-changing early warning of COVID-19 infections."
The presence of the SARS-CoV-2 pathogen can be detected in human waste of infected individuals – even in asymptomatic or presymptomatic patients. LuminUltra's wastewater testing solution utilizes the rapid and portable GeneCount® qPCR device, the same industry gold-standard technology used in clinical diagnostic testing. While other solutions around the world can take days or weeks and require specialized lab expertise to analyze a mailed-in sample, LuminUltra's solution examines multiple samples on-site within 90 minutes, without the need for specific testing expertise – making the testing process more efficient and therefore available at a lower price point than other wastewater testing solutions.
"Public health leaders around the world have validated that wastewater testing is a powerful tool in the fight against the pandemic, and global research leaders have demonstrated the benefits of testing human waste in controlled populations," said Dr. Amina Stoddart of Dalhousie University. "Wastewater testing has been shown to lead to early identification of the virus before it is known in a clinical context – the potential benefit could help Public Health leaders with additional information for decisions concerning the pandemic. We are very pleased to continue Dalhousie's long-standing research relationship with LuminUltra to help fight the COVID-19 pandemic in Halifax and beyond."
"Halifax Water is proud to be a partner in this important wastewater research which aligns well with our focus on public health and environmental protection. This collaboration is an extension of our history of supporting and fostering innovative research in water and wastewater," said General Manager Cathie O'Toole, of Halifax Water. "Halifax and other communities are looking for monitoring solutions to reliably gather information about population health and effectively control the spread of COVID-19."
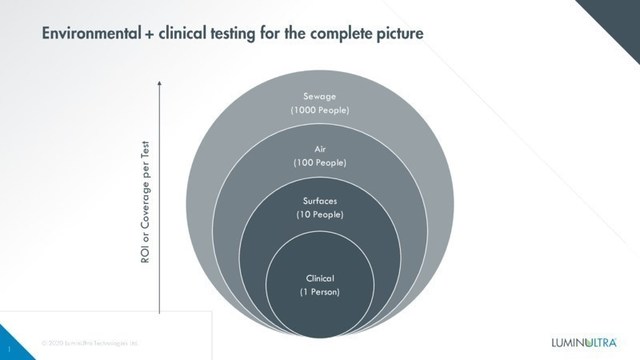
LuminUltra's wastewater testing solution allows communities and controlled populations to analyze overall population health, rather than relying solely on single-patient clinical tests to determine if there is an infection of COVID-19 present or a surge in cases. Currently, North America does not have the capacity or resources to control the spread of COVID-19 through clinical testing alone. With 40 per cent of infected COVID-19 patients being asymptomatici, and with clinical testing kits being in high demand with concern of a pending shortage, a smarter, broader, more holistic testing approach is needed. LuminUltra is pleased to bring a reliable, trusted solution to communities all over the world.
COVID-19 Testing Solutions
While individual human carriers are tested for COVID-19 using clinical diagnostics, broader population-based testing is also essential to identify the presence of SARS-CoV-2 within a community or environment. LuminUltra provides complete testing solutions for each of the three protocols: clinical diagnosticsii, surface and air testing and now, wastewater testing.
The GeneCount qPCR devices include both the portable and high-capacity options. They can be used to run both the clinical and environment tests to provide a holistic and efficient approach to pandemic management.
About LuminUltra
Founded in 1995, LuminUltra is a biological diagnostic testing company headquartered in Canada with operations in 6 countries. It is widely recognized globally as a leader in developing tests and reagents for environmental, industrial, and diagnostic monitoring and is a key supplier of COVID-19 clinical testing reagents to the Government of Canada. Customers in over 80 countries trust LuminUltra's technology, production reliability and history of customer service excellence to deliver their essential services in a safe-state. At the same time, LuminUltra fosters a culture of innovation and agility and is on an accelerated growth path, acquiring multiple companies in recent years and forming a partnership with the specialized private equity firm XPV Water Partners. Additional information can be found at luminultra.com.
i https://www.cdc.gov/coronavirus/2019-ncov/hcp/planning-scenarios.html
ii GeneCount® COVID-19 RT-qPCR Assay Kit pending FDA and Health Canada approval
View original content to download multimedia:http://www.prnewswire.com/news-releases/luminultra-files-patent-for-the-worlds-first-rapid-on-site-covid-19-wastewater-testing-solution-301156908.html
SOURCE LuminUltra