Infectious disease
Studies are also continuing on the value and efficacy of booster shots, particularly against the Delta variant. Read on for more information.
The report also highlighted that an unvaccinated individual is five times more likely to get COVID-19 than someone who is fully vaccinated.
Shares of Tonix Pharmaceuticals were up nearly 11% in premarket trading after the company announced plans to initiate a Phase II study of TNX-102 SL as a potential treatment for Long COVID Syndrome following a positive meeting with the U.S. FDA.
President Biden is expected to be briefed on the lab leak theory conducted over the last 90 days. Very few expect solid results, although this might provide direction for more lines of inquiry.
The new authorization is expected to be the final reassurance for many of those who had hesitated when the vaccine was approved under emergency use only.
An oral version of remdesivir is being developed by scientists at the University of California San Diego to combat the SARS-CoV-2 virus early in its infection cycle.
While last flu season saw fears of a “twindemic” go largely unfounded, there are fears that recent lockdowns could also lead to a lack of natural immunity against the flu.
Research presented earlier this month discussed a possible link between COVID-19 and long-term cognitive deficits and how it may accelerate the pathology and symptoms of Alzheimer’s disease.
Many expect the full approval will lead to vaccine mandates, as that was one of the obstacles to it for many organizations.
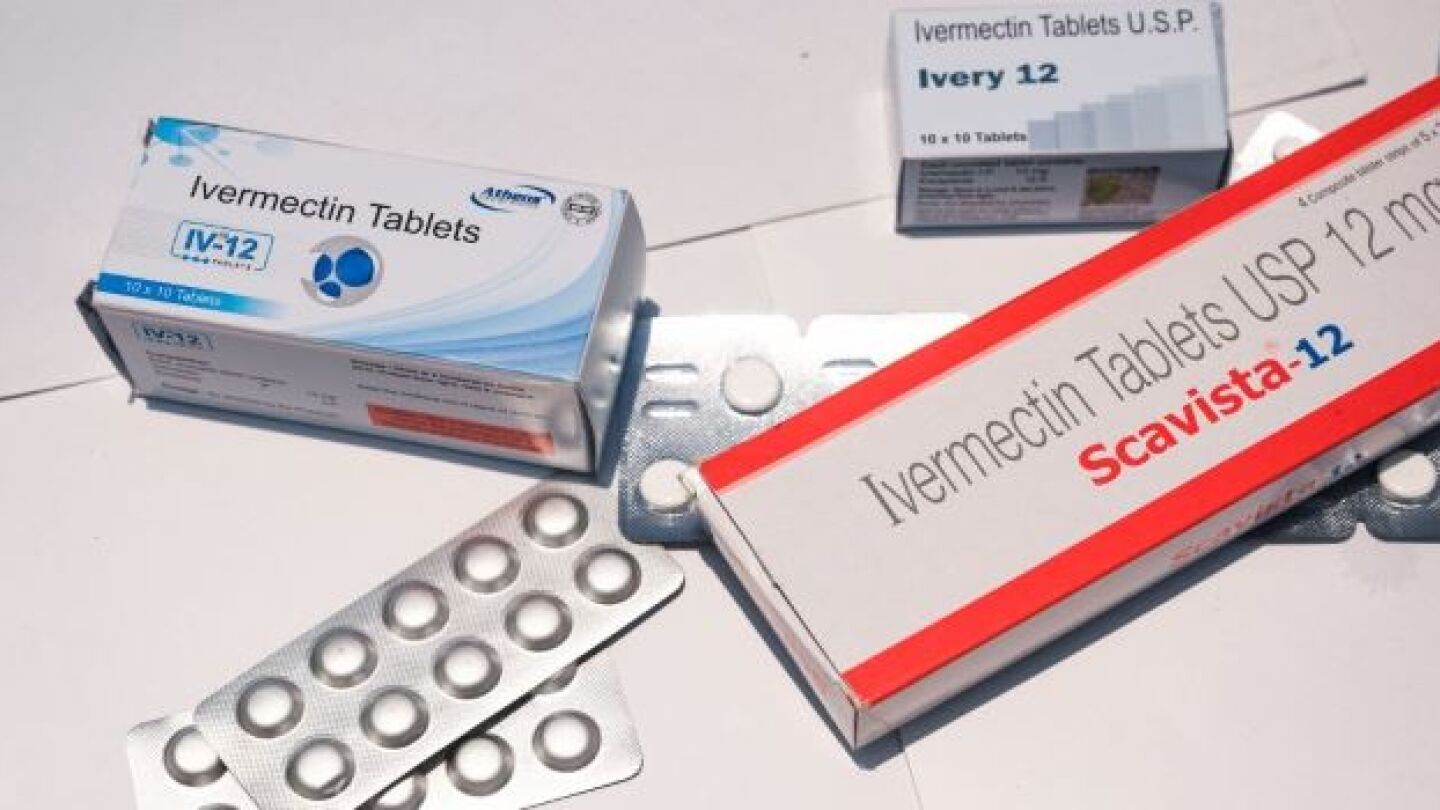
“You are not a horse. You are not a cow. Seriously, y’all. Stop it.” This is the FDA’s recently tweeted advice to Americans seeking out alternative, unapproved treatments for COVID-19.
PRESS RELEASES