Featured
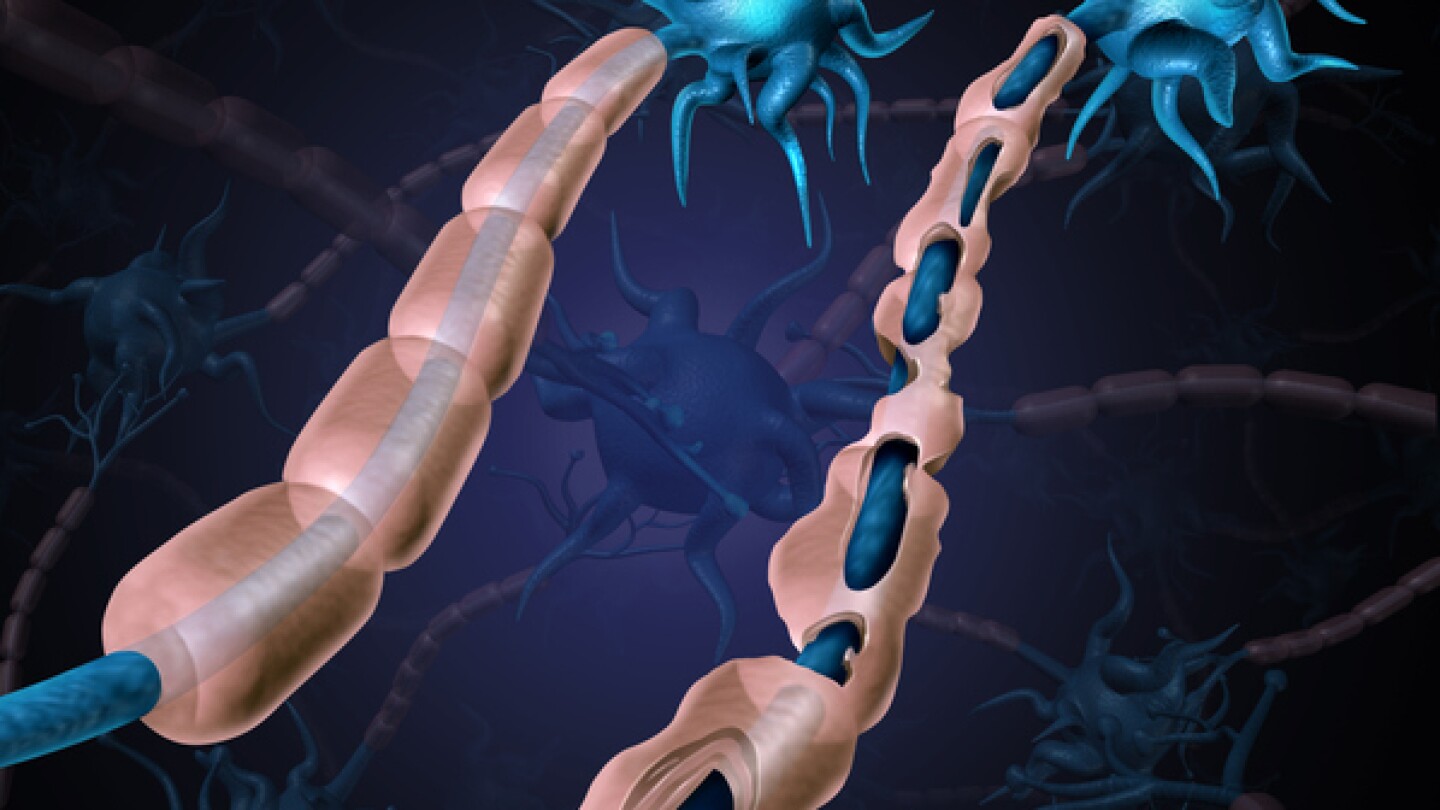
While Bruton’s tyrosine kinase inhibitors are often hailed as the next big breakthrough in multiple sclerosis, Immunic Therapeutics and others are leveraging neuroprotective targets and remyelination to keep the disease at bay.
Following up on previous, dimly received issuances, a new set of ideas published by the FDA to streamline regulatory pathways for cell and gene therapies ‘for small populations’ is receiving a warmer welcome—but experts warn it will take more to turn the tide for the fraught therapeutic space.
J&J still holds the top deal of the year by value with its $14.6 billion buy of Intra-Cellular in January, but the next four biggest acquisitions came in the past four months.
The two most historically deal-conservative Big Pharmas have the most money to play with for a major M&A transaction, according to a recent Stifel analysis.
A new analysis from SRS Acquiom puts into perspective the headline values seen when a company announces a backloaded M&A deal. Biotechs have much on the line when they agree to deals with massive potential but little upfront.
Talks between pharma and successive U.K. governments have failed to deliver the market access terms that the industry wants, contributing to a pullback in investment.
Companies are moving from using AI for distinct operations to applying the technology for control and optimization of the whole production process.
Reshoring generic pharmaceutical production is essential in today’s era of geopolitical instability and heightened awareness surrounding national health security. And it is possible—if done right.
The FDA in September issued two rejections for spinal muscular atrophy therapies—both linked to manufacturing problems—and granted approvals in Barth syndrome and for a subcutaneous version of Merck’s Keytruda that could be key to the blockbuster’s future earnings.
By publishing complete response letters as soon as they are issued to drug sponsors, the FDA is expanding transparency in a way that, while positioned as a public health measure, also grants investors greater visibility into regulatory decisions. Experts question whether this is the agency’s proper remit.