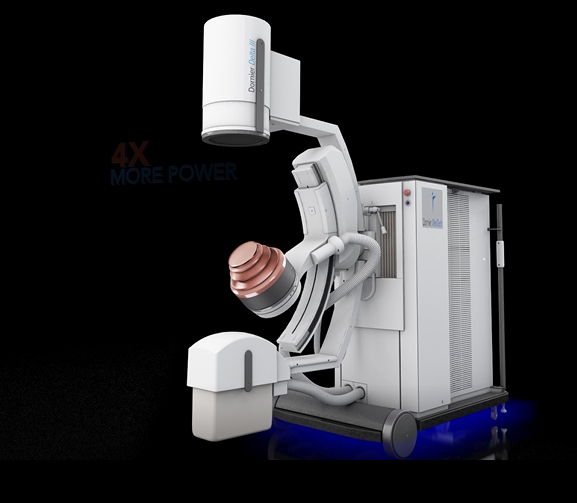
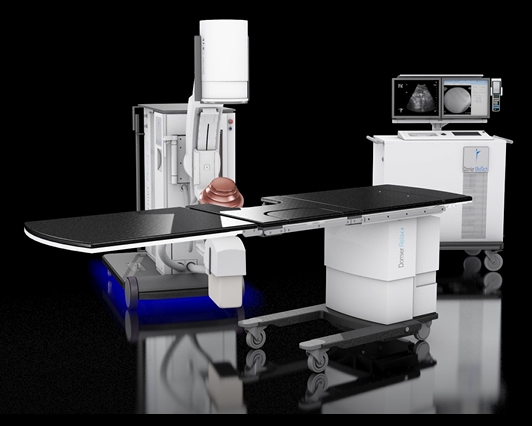
ATLANTA, July 11, 2017 /PRNewswire/ -- Dornier MedTech® America, a global medical device company and the pioneer of Extracorporeal Shock Wave Lithotripsy (ESWL®), has received FDA clearance to market the Delta® III. This is the latest generation of the world's best-selling and most clinically cited lithotripter, the Delta II.
Experience the interactive Multichannel News Release here: https://www.multivu.com/players/English/8134451-dornier-medtech-delta-iii-kidney-stone-lithotripter/
The Delta III reflects Dornier's continued commitment to developing the most cutting-edge solutions for kidney stone treatment. Compared to its predecessor, the Delta III provides improvements of:
- Powerful IMAGING (for improved stone visualization)
- Maximized ENERGY (to treat more stones in more patients)
- Enhanced EFFICIENCY (with time saving features to serve more patients)
Powerful IMAGING: the system provides improved imaging quality with 400%2 more power and two imaging modalities (Ultrasound or X-ray) for better stone visibility.
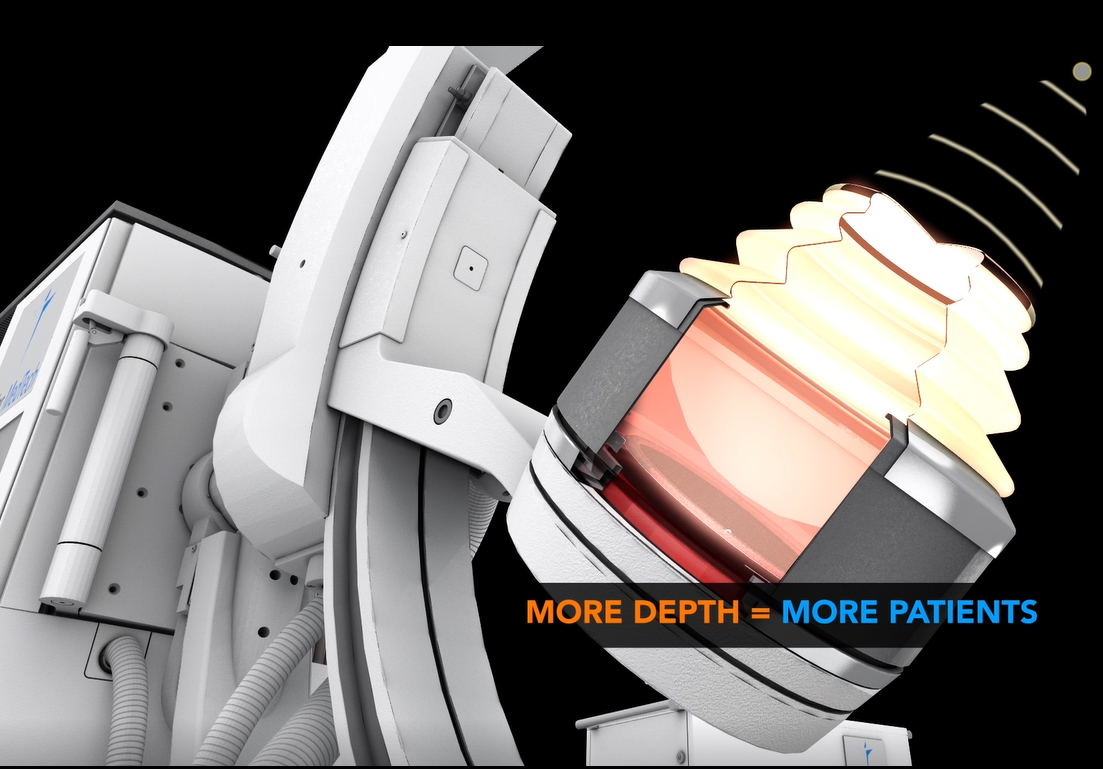
Maximized ENERGY: the New 180 EMSE (+25% larger2) delivers more power for enhanced penetration depth to treat larger patients. In a 183 patient clinical study, the new 180 EMSE was shown to deliver a similar 95% stone free success rate for patients with BMI higher than 25, as with patients with BMI below 25.3 Combined with Dornier's exclusive Opticouple®, an optical camera system, visualized air bubbles are removed in the coupling gel at the interface of the therapy head and patient's skin via the user. Clinical research4 shows a 43% increase in effectiveness with less shock waves energy needed per treatment.
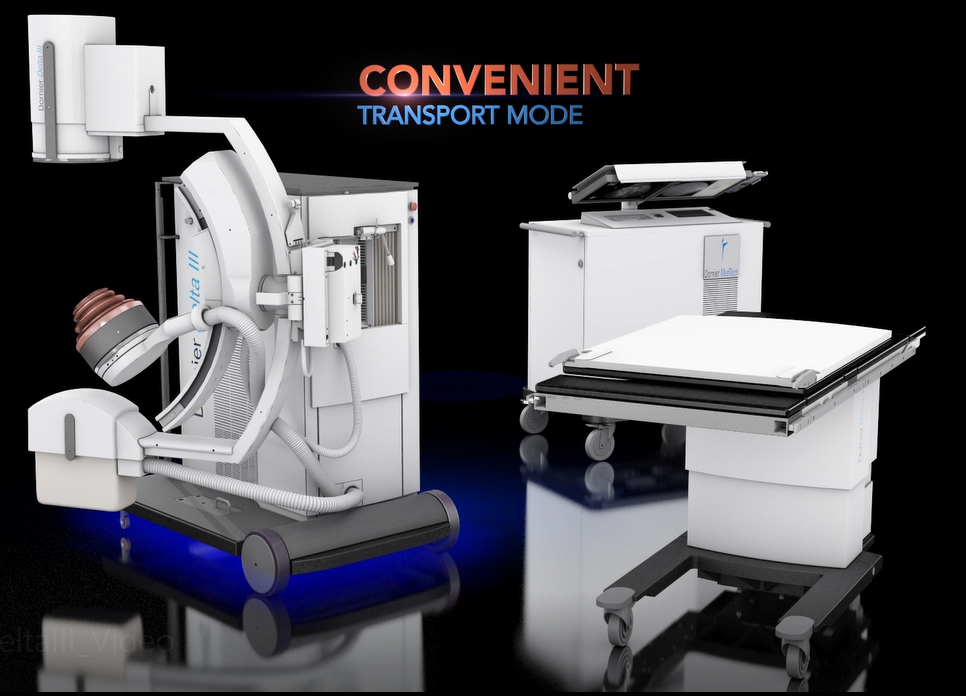
Enhanced EFFICIENCY: Additionally, the Delta III increases ease of use and improves time saving workflow with its patent protected Unified Hand Control with precise fingertip control of the lithotripter, table and X-ray C-arm movements.
The Delta® III is proudly made in Munich, Germany.
To learn more click below:
https://youtu.be/RyIFy32OaI4
Dornier MedTech, a wholly-owned subsidiary of Accuron Medical Technology group (www.accuron.com). Dornier MedTech is focused on delivering scientifically superior products and solutions to physicians and patients involved in urological care. As pioneers of ESWL®, lithotripsy and surgical lasers, Dornier's 40 years of innovation and service has made it one of the most trusted MedTech companies in the industry. Dornier MedTech is headquartered in Munich, Germany with offices and distributors all over the world. For more information, visit www.dornier.com.
1http://www.kidneystoners.org/wp-content/uploads/2012/01/comparing-kidney-stone-surgeries_1-12-12.jpg
2 As compared to the Compact Delta® II
3 M. Mohammadi, T. Au, N. Milz, S. Osswald, A. Zintl, H. P. Bastian, G. Lümmen, St. Josef-Hospital Troisdorf Study Hospital: "The Clinical Experience with the New Dornier Shock Wave Source EMSE 180 for ESWL", Düsseldorf, 60 Congress of the North Rhine-Westphalian Society of Urology, (April 3 4, 2014).
4 Tailly GG, Tailly-Cusse MM: "Optical Coupling Control: An Important Step Toward Better Lithotripsy", J Endourol (2014 28(11):1368-73).
©2017 DORNIER MEDTECH. All rights reserved. Subject to change without notice. Delta®, Opticouple® and ESWL® are registered trademarks of Dornier MedTech. All subsequent uses of these brand names throughout this document are also protected.
LinkedIn Twitter YouTube
http://www.twitter.com/DornierMedTech
https://www.linkedin.com/company/dornier-medtech
https://www.youtube.com/user/DornierMedTechVideo

View original content:http://www.prnewswire.com/news-releases/dornier-medtech-launches-the-dornier-delta-iii--most-advanced-kidney-stone-treatment-lithotripter-300486336.html
SOURCE Dornier MedTech

