All News
With the biopharma industry’s looming wave of gene therapy submissions and potential approvals, the senior senator is laying the groundwork for a legislative initiative to improve access to these expensive treatments.
In two late-stage trials, the experimental oral drug evobrutinib was unable to significantly reduce annualized relapse rates in MS patients compared with Sanofi’s Aubagio tablets.
Fueled partly by increasing lifespans and cancer incidence, the area’s worth is projected to soar to 13.67 billion by 2032, Precedence Research reports.
A combination of Roche’s investigational treatment with Ibrance and fulvestrant met its primary endpoint of progression-free survival in the first-line setting in treating PIK3CA-mutated breast cancer.
Ten of the anticipated assets have the potential to deliver $5 billion or more in peak year sales, while another 15 of the candidates have a $1 billion to $5 billion PYS potential, according to Johnson & Johnson.
In this third episode of Denatured’s series on AI in drug discovery, we discuss patient behavior and its influence on clinical trials and AI models with guests from GSK, IQVIA, Exelixis and DataHow.
The gene editing company is dropping two programs and favoring its next-generation assets CTX112 and CTX131, which it will continue to develop in oncology but will also test in autoimmune diseases.
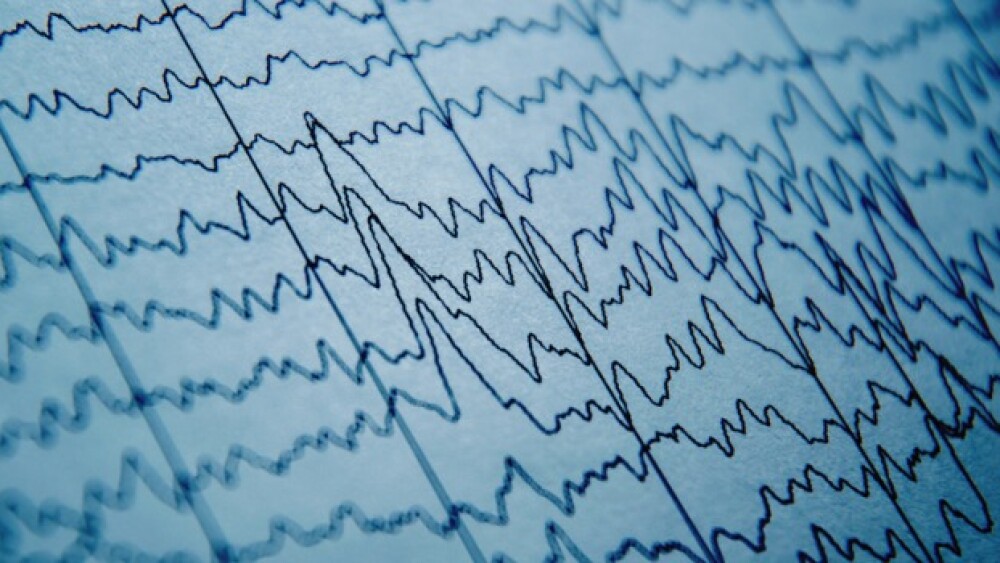
ALTO-300 was significantly more effective in major depressive disorder patients with a specific EEG biomarker than in those without, according to results from an Alto Neuroscience Phase IIa study.
The regulator placed a partial clinical hold on Roche’s fenebrutinib—being developed for relapsing MS—after two patients experienced elevated hepatic transaminase and bilirubin levels indicative of liver injury.
The pharma industry serves patients best when data is shared, and privacy concerns can stand in the way of resolving critical supply chain issues.
EyePoint Pharmaceuticals’ treatment for wet age-related macular degeneration showed comparable results to Regeneron’s Eylea with a less frequent dosing regimen.
Mass.-based Seismic Therapeutic secured a new investor in Bessemer Venture Partners and $121 million in its Series B round as it looks to push its two lead candidates into the clinic.
In the latest example of big pharma entering AI collaborations or using AI-based tools, AstraZeneca’s deal with Absci will aim to produce an oncology candidate.
The acquisition will give Roche access to Carmot’s clinical portfolio of three GLP-1 receptor agonists, placing it squarely in the middle of the competition to treat overweight and obesity.
The European Medicines Agency is seeking additional information from the makers of GLP-1 drugs as part of its ongoing review of the potential risk of suicide and self-harm thoughts associated with the class.
Lilly’s Jaypirca (pirtobrutinib) can now be used to treat chronic lymphocytic leukemia or small lymphocytic leukemia that had progressed from at least two prior lines of therapy, in addition to its previously-approved indications.
Johnson & Johnson’s AI investments include a research facility in San Francisco and a data science workforce of approximately 6,000 employees.
AbbVie’s $10.1 billion ImmunoGen buy and Altimmune’s Phase II win demonstrate that the antibody-drug conjugate market is red hot in cancer and GLP-1 drugs for weight loss are an absolute craze.
While Pfizer’s oral GLP-1 candidate met its primary endpoint in a Phase IIb obesity trial, twice-daily dosing of danuglipron resulted in high rates of adverse events including nausea, vomiting and diarrhea.
From the skin to the lungs to the central nervous system, biotech companies are making progress toward delivering RNA therapeutics to multiple targets throughout the body. But challenges remain.