Influenza B Market Outlook 2024-2034:
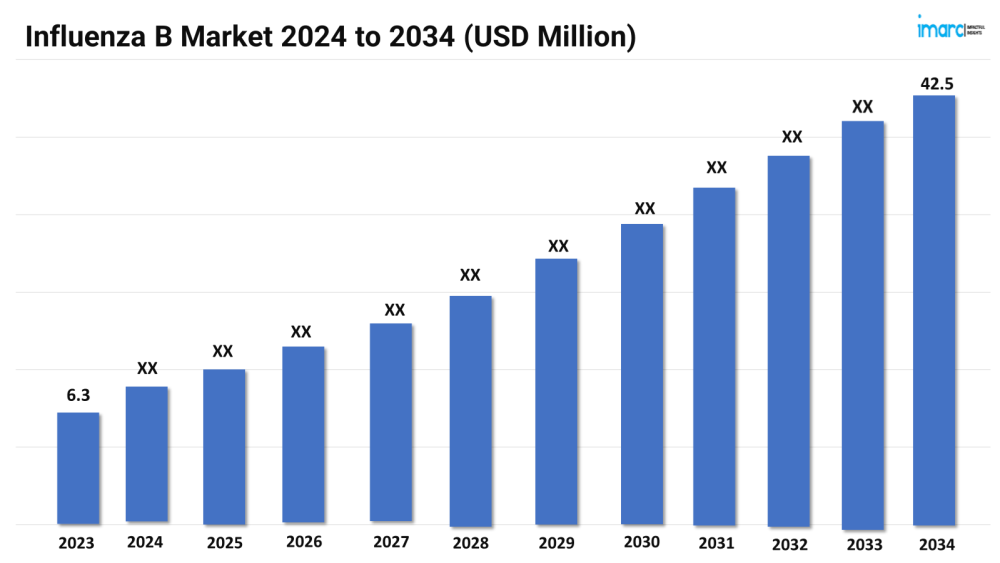
The influenza B market size reached a value of USD 6.3 Million in 2023. Looking forward, the market is expected to reach USD 42.5 Million by 2034, exhibiting a growth rate (CAGR) of 19% during 2024-2034.
The market is driven by innovative vaccine developments and improved antiviral therapies. Additionally, there is a growing emphasis on universal flu vaccines aiming to provide broader and longer-lasting protection.
Adoption of Quadrivalent Vaccines: Driving the Influenza B Market
The introduction of quadrivalent vaccines is a watershed moment in the influenza B market, dramatically increasing the efficacy of flu prevention efforts. Unlike typical trivalent vaccines, which protect against three influenza strains (two A strains and one B strain), quadrivalent vaccines also protect against an additional B strain. This larger coverage is critical because Influenza B viruses circulate alongside Influenza A viruses each year, and their incidence varies seasonally. Quadrivalent vaccines, which include both B strains (Victoria and Yamagata lineages), provide more comprehensive protection, lowering the chance of mismatched strains and enhancing overall vaccine effectiveness.
Request a PDF Sample Report: https://www.imarcgroup.com/influenza-b-market/requestsample
One notable instance of this trend is the widespread adoption of quadrivalent vaccines by public health organizations and healthcare providers. For example, the Centers for Disease Control and Prevention in the United States recommends the use of quadrivalent vaccines for all eligible individuals aged six months and older. Companies like Sanofi Pasteur, with their Fluzone Quadrivalent, and GlaxoSmithKline, with their Fluarix Quadrivalent, have led the market with these enhanced vaccines. Additionally, the introduction of cell-based and recombinant quadrivalent vaccines, such as Seqirus’ Flucelvax Quadrivalent and Sanofi's Flublok Quadrivalent, represents a significant advancement. These newer vaccines avoid the limitations associated with egg-based production, such as egg allergy risks and longer production times, offering potentially higher effectiveness and faster scalability in response to flu outbreaks. The shift towards quadrivalent vaccines is also supported by increasing public health awareness and educational campaigns emphasizing the importance of broad-spectrum flu protection. As a result, vaccination coverage rates have improved, contributing to better management of influenza seasons and reducing the incidence of flu-related complications and hospitalizations. The ongoing focus on research and development by pharmaceutical companies and the support from public health agencies underscore the commitment to enhancing influenza prevention strategies, ultimately aiming to mitigate the impact of influenza B and other strains on public health.
Advancements in Vaccine Technologies: Contributing to Market Expansion
Influenza B is a contagious respiratory illness due to the Influenza B virus, which primarily affects humans and is responsible for significant seasonal outbreaks worldwide. Unlike Influenza A, which can infect a wide range of species and lead to pandemics, Influenza B is typically limited to humans, reducing its pandemic potential. The virus has two main lineages, B/Yamagata and B/Victoria, and vaccines are formulated to provide protection against both. These vaccines are crucial in preventing severe illness, hospitalizations, and deaths associated with Influenza B. They work by stimulating the body's immune system to produce antibodies against the virus, thereby reducing the risk of infection and mitigating the acuteness of symptoms in those who do contract the virus. Influenza B vaccines are particularly important for vulnerable populations, including the elderly, young children, and individuals with chronic health conditions.
Advancements in vaccine technologies have significantly enhanced the effectiveness and accessibility of Influenza B vaccines. A notable development is the introduction of cell-based and recombinant vaccine production methods. Traditional flu vaccines are produced using chicken eggs, a process that can be slow and less efficient. Cell-based vaccines, such as Flucelvax, use cultured mammalian cells, which allow for faster and more flexible production, particularly in the face of sudden outbreaks. Recombinant vaccines, like Flublok, utilize genetic engineering to produce the hemagglutinin protein, a key component of the virus, in insect cells, bypassing the need for eggs entirely. These modern techniques not only expedite vaccine production but also offer an option for those with egg allergies. Additionally, the development of quadrivalent vaccines, which protect against four different flu virus strains, including both lineages of Influenza B, represents a significant advancement over trivalent vaccines that cover only three strains. The continuous evolution of these technologies underscores a robust and adaptive approach to combating Influenza B, ensuring broader protection and improved public health outcomes.
Development of Universal Flu Vaccines:
Influenza B is a significant contributor to seasonal flu epidemics, causing a substantial burden on public health systems globally. Unlike Influenza A, which is known for its ability to cause pandemics due to its broad host range and higher mutation rates, Influenza B primarily affects humans and tends to cause less severe outbreaks. However, it still poses a serious threat, particularly to vulnerable populations such as children, the elderly, and those with underlying health conditions. The current vaccination strategy involves annually updated vaccines that target the predicted strains of the virus. Despite these efforts, the virus’s ability to mutate frequently results in mismatches between vaccine strains and circulating strains, reducing vaccine effectiveness. Consequently, there is a growing need for more reliable and long-lasting solutions to combat Influenza B.
The development of universal flu vaccines aims to address this challenge by providing broad and long-lasting protection against multiple influenza strains, including both Influenza A and B. One promising approach involves targeting the conserved regions of the virus that remain relatively balanced across different strains. For instance, researchers are focusing on the hemagglutinin (HA) stalk region, which is less variable than the head region targeted by traditional vaccines. Studies have shown that vaccines targeting the HA stalk can induce broadly protective immune responses. Another innovative strategy is the use of nanoparticle-based vaccines, such as those developed by the University of Washington’s Institute for Protein Design, which presents multiple viral antigens in a highly immunogenic format. Clinical trials for these universal vaccines are underway, with some showing promising results in early-phase trials. The successful introduction of a universal flu vaccine would represent a paradigm shift in influenza prevention, potentially eliminating the need for annual vaccine updates and providing consistent protection against both seasonal and pandemic influenza strains. This advancement could significantly reduce the global burden of influenza, improving health outcomes and reducing healthcare costs associated with flu-related illnesses.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8714&method=587
Leading Companies in the Influenza B Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global influenza B market, several leading pharmaceutical companies play a major role in the development, production, and distribution of influenza vaccines. Some of the major players include GlaxoSmithKline, AstraZeneca, and Sanofi These companies are at the forefront of innovation, striving to improve vaccine efficacy, production processes, and accessibility.
GlaxoSmithKline has also made significant strides with Hiberix, its Haemophilus influenzae type b (Hib) conjugate vaccine. Hiberix is essential in protecting against invasive diseases caused by Hib bacteria, such as meningitis, pneumonia, and epiglottitis, which predominantly affect young children.
Moreover, AstraZeneca has made significant advancements with FluMist. The company announced new data supporting the effectiveness and safety of FluMist Quadrivalent, which has led to updated recommendations and approvals in various regions.
Apart from this, Sanofi is expanding its production capabilities and distribution networks to ensure broader access to Fluzone, particularly in underserved regions. The company's efforts include collaborations with global health organizations to improve vaccination rates and protect vulnerable populations worldwide.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8714&flag=E
Regional Analysis:
The major markets for influenza B include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for influenza B while also representing the biggest market for its treatment. This can be attributed to the focus on high-risk populations and the research and development of universal flu vaccines.
Moreover, there is a heightened focus on targeting high-risk populations with specific vaccine formulations. For instance, Sanofi's Fluzone High-Dose is tailored for the elderly, who are more susceptible to severe flu complications. This formulation contains a higher antigen content to elicit a stronger immune response, thereby providing better protection for older adults.
Besides this, a significant long-term trend is the research and development of universal flu vaccines. These vaccines aim to provide broad and long-lasting protection against multiple influenza strains, including both Influenza A and B. Efforts by organizations such as the National Institutes of Health (NIH) and various biotech firms are focused on identifying conserved viral components that can induce a more universal immune response.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the influenza B market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the influenza B market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current influenza B marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/influenza-b-market
IMARC Group Offer Other Reports:
Macular Telangiectasia Market: The 7 major macular telangiectasia market is expected to exhibit a CAGR of 6.7% during the forecast period from 2024 to 2034.
Long qt Syndrome Market: The 7 major long qt syndrome market is expected to exhibit a CAGR of 5.1% during the forecast period from 2024 to 2034.
Skin Neoplasms Market: The 7 major skin neoplasms market reached a value of US$ 2.2 Billion in 2023, and projected the 7MM to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 4.91% during the forecast period from 2024 to 2034.
Cluster Headache Market: The 7 major cluster headache market reached a value of US$ 812.8 Million in 2023, and projected the 7MM to reach US$ 1,124.6 Million by 2034, exhibiting a growth rate (CAGR) of 3% during the forecast period from 2024 to 2034.
Malignant Mesothelioma Market: The 7 major malignant mesothelioma market reached a value of US$ 6.1 Billion in 2023, and projected the 7MM to reach US$ 12.2 Billion by 2034, exhibiting a growth rate (CAGR) of 6.5% during the forecast period from 2024 to 2034.
Desmoid Tumors Market: The 7 major desmoid tumors market reached a value of US$ 1.7 Billion in 2023, and projected the 7MM to reach US$ 3.2 Billion by 2034, exhibiting a growth rate (CAGR) of 5.61% during the forecast period from 2024 to 2034.
Female Infertility Market: The 7 major female infertility market reached a value of US$ 2.0 Billion in 2023, and projected the 7MM to reach US$ 2.7 Billion by 2034, exhibiting a growth rate (CAGR) of 2.76%during the forecast period from 2024-2034.
Filariasis Market: The 7 major filariasis market is expected to exhibit a CAGR of 7.92% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800
The influenza B market size reached a value of USD 6.3 Million in 2023. Looking forward, the market is expected to reach USD 42.5 Million by 2034, exhibiting a growth rate (CAGR) of 19% during 2024-2034.
The market is driven by innovative vaccine developments and improved antiviral therapies. Additionally, there is a growing emphasis on universal flu vaccines aiming to provide broader and longer-lasting protection.
Adoption of Quadrivalent Vaccines: Driving the Influenza B Market
The introduction of quadrivalent vaccines is a watershed moment in the influenza B market, dramatically increasing the efficacy of flu prevention efforts. Unlike typical trivalent vaccines, which protect against three influenza strains (two A strains and one B strain), quadrivalent vaccines also protect against an additional B strain. This larger coverage is critical because Influenza B viruses circulate alongside Influenza A viruses each year, and their incidence varies seasonally. Quadrivalent vaccines, which include both B strains (Victoria and Yamagata lineages), provide more comprehensive protection, lowering the chance of mismatched strains and enhancing overall vaccine effectiveness.
Request a PDF Sample Report: https://www.imarcgroup.com/influenza-b-market/requestsample
One notable instance of this trend is the widespread adoption of quadrivalent vaccines by public health organizations and healthcare providers. For example, the Centers for Disease Control and Prevention in the United States recommends the use of quadrivalent vaccines for all eligible individuals aged six months and older. Companies like Sanofi Pasteur, with their Fluzone Quadrivalent, and GlaxoSmithKline, with their Fluarix Quadrivalent, have led the market with these enhanced vaccines. Additionally, the introduction of cell-based and recombinant quadrivalent vaccines, such as Seqirus’ Flucelvax Quadrivalent and Sanofi's Flublok Quadrivalent, represents a significant advancement. These newer vaccines avoid the limitations associated with egg-based production, such as egg allergy risks and longer production times, offering potentially higher effectiveness and faster scalability in response to flu outbreaks. The shift towards quadrivalent vaccines is also supported by increasing public health awareness and educational campaigns emphasizing the importance of broad-spectrum flu protection. As a result, vaccination coverage rates have improved, contributing to better management of influenza seasons and reducing the incidence of flu-related complications and hospitalizations. The ongoing focus on research and development by pharmaceutical companies and the support from public health agencies underscore the commitment to enhancing influenza prevention strategies, ultimately aiming to mitigate the impact of influenza B and other strains on public health.
Advancements in Vaccine Technologies: Contributing to Market Expansion
Influenza B is a contagious respiratory illness due to the Influenza B virus, which primarily affects humans and is responsible for significant seasonal outbreaks worldwide. Unlike Influenza A, which can infect a wide range of species and lead to pandemics, Influenza B is typically limited to humans, reducing its pandemic potential. The virus has two main lineages, B/Yamagata and B/Victoria, and vaccines are formulated to provide protection against both. These vaccines are crucial in preventing severe illness, hospitalizations, and deaths associated with Influenza B. They work by stimulating the body's immune system to produce antibodies against the virus, thereby reducing the risk of infection and mitigating the acuteness of symptoms in those who do contract the virus. Influenza B vaccines are particularly important for vulnerable populations, including the elderly, young children, and individuals with chronic health conditions.
Advancements in vaccine technologies have significantly enhanced the effectiveness and accessibility of Influenza B vaccines. A notable development is the introduction of cell-based and recombinant vaccine production methods. Traditional flu vaccines are produced using chicken eggs, a process that can be slow and less efficient. Cell-based vaccines, such as Flucelvax, use cultured mammalian cells, which allow for faster and more flexible production, particularly in the face of sudden outbreaks. Recombinant vaccines, like Flublok, utilize genetic engineering to produce the hemagglutinin protein, a key component of the virus, in insect cells, bypassing the need for eggs entirely. These modern techniques not only expedite vaccine production but also offer an option for those with egg allergies. Additionally, the development of quadrivalent vaccines, which protect against four different flu virus strains, including both lineages of Influenza B, represents a significant advancement over trivalent vaccines that cover only three strains. The continuous evolution of these technologies underscores a robust and adaptive approach to combating Influenza B, ensuring broader protection and improved public health outcomes.
Development of Universal Flu Vaccines:
Influenza B is a significant contributor to seasonal flu epidemics, causing a substantial burden on public health systems globally. Unlike Influenza A, which is known for its ability to cause pandemics due to its broad host range and higher mutation rates, Influenza B primarily affects humans and tends to cause less severe outbreaks. However, it still poses a serious threat, particularly to vulnerable populations such as children, the elderly, and those with underlying health conditions. The current vaccination strategy involves annually updated vaccines that target the predicted strains of the virus. Despite these efforts, the virus’s ability to mutate frequently results in mismatches between vaccine strains and circulating strains, reducing vaccine effectiveness. Consequently, there is a growing need for more reliable and long-lasting solutions to combat Influenza B.
The development of universal flu vaccines aims to address this challenge by providing broad and long-lasting protection against multiple influenza strains, including both Influenza A and B. One promising approach involves targeting the conserved regions of the virus that remain relatively balanced across different strains. For instance, researchers are focusing on the hemagglutinin (HA) stalk region, which is less variable than the head region targeted by traditional vaccines. Studies have shown that vaccines targeting the HA stalk can induce broadly protective immune responses. Another innovative strategy is the use of nanoparticle-based vaccines, such as those developed by the University of Washington’s Institute for Protein Design, which presents multiple viral antigens in a highly immunogenic format. Clinical trials for these universal vaccines are underway, with some showing promising results in early-phase trials. The successful introduction of a universal flu vaccine would represent a paradigm shift in influenza prevention, potentially eliminating the need for annual vaccine updates and providing consistent protection against both seasonal and pandemic influenza strains. This advancement could significantly reduce the global burden of influenza, improving health outcomes and reducing healthcare costs associated with flu-related illnesses.
Buy Full Report: https://www.imarcgroup.com/checkout?id=8714&method=587
Leading Companies in the Influenza B Market:
The market research report by IMARC encompasses a comprehensive analysis of the competitive landscape in the market. Across the global influenza B market, several leading pharmaceutical companies play a major role in the development, production, and distribution of influenza vaccines. Some of the major players include GlaxoSmithKline, AstraZeneca, and Sanofi These companies are at the forefront of innovation, striving to improve vaccine efficacy, production processes, and accessibility.
GlaxoSmithKline has also made significant strides with Hiberix, its Haemophilus influenzae type b (Hib) conjugate vaccine. Hiberix is essential in protecting against invasive diseases caused by Hib bacteria, such as meningitis, pneumonia, and epiglottitis, which predominantly affect young children.
Moreover, AstraZeneca has made significant advancements with FluMist. The company announced new data supporting the effectiveness and safety of FluMist Quadrivalent, which has led to updated recommendations and approvals in various regions.
Apart from this, Sanofi is expanding its production capabilities and distribution networks to ensure broader access to Fluzone, particularly in underserved regions. The company's efforts include collaborations with global health organizations to improve vaccination rates and protect vulnerable populations worldwide.
Request for customization: https://www.imarcgroup.com/request?type=report&id=8714&flag=E
Regional Analysis:
The major markets for influenza B include the United States, Germany, France, the United Kingdom, Italy, Spain, and Japan. According to projections by IMARC, the United States has the largest patient pool for influenza B while also representing the biggest market for its treatment. This can be attributed to the focus on high-risk populations and the research and development of universal flu vaccines.
Moreover, there is a heightened focus on targeting high-risk populations with specific vaccine formulations. For instance, Sanofi's Fluzone High-Dose is tailored for the elderly, who are more susceptible to severe flu complications. This formulation contains a higher antigen content to elicit a stronger immune response, thereby providing better protection for older adults.
Besides this, a significant long-term trend is the research and development of universal flu vaccines. These vaccines aim to provide broad and long-lasting protection against multiple influenza strains, including both Influenza A and B. Efforts by organizations such as the National Institutes of Health (NIH) and various biotech firms are focused on identifying conserved viral components that can induce a more universal immune response.
Key information covered in the report.
Base Year: 2023
Historical Period: 2018-2023
Market Forecast: 2024-2034
Countries Covered
· United States
· Germany
· France
· United Kingdom
· Italy
· Spain
· Japan
Analysis Covered Across Each Country
· Historical, current, and future epidemiology scenario
· Historical, current, and future performance of the influenza B market
· Historical, current, and future performance of various therapeutic categories in the market
· Sales of various drugs across the influenza B market
· Reimbursement scenario in the market
· In-market and pipeline drugs
Competitive Landscape:
This report offers a comprehensive analysis of current influenza B marketed drugs and late-stage pipeline drugs.
In-Market Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Late-Stage Pipeline Drugs
· Drug Overview
· Mechanism of Action
· Regulatory Status
· Clinical Trial Results
· Drug Uptake and Market Performance
Ask Our Expert & Browse Full Report with TOC & List of Figure: https://www.imarcgroup.com/influenza-b-market
IMARC Group Offer Other Reports:
Macular Telangiectasia Market: The 7 major macular telangiectasia market is expected to exhibit a CAGR of 6.7% during the forecast period from 2024 to 2034.
Long qt Syndrome Market: The 7 major long qt syndrome market is expected to exhibit a CAGR of 5.1% during the forecast period from 2024 to 2034.
Skin Neoplasms Market: The 7 major skin neoplasms market reached a value of US$ 2.2 Billion in 2023, and projected the 7MM to reach US$ 3.8 Billion by 2034, exhibiting a growth rate (CAGR) of 4.91% during the forecast period from 2024 to 2034.
Cluster Headache Market: The 7 major cluster headache market reached a value of US$ 812.8 Million in 2023, and projected the 7MM to reach US$ 1,124.6 Million by 2034, exhibiting a growth rate (CAGR) of 3% during the forecast period from 2024 to 2034.
Malignant Mesothelioma Market: The 7 major malignant mesothelioma market reached a value of US$ 6.1 Billion in 2023, and projected the 7MM to reach US$ 12.2 Billion by 2034, exhibiting a growth rate (CAGR) of 6.5% during the forecast period from 2024 to 2034.
Desmoid Tumors Market: The 7 major desmoid tumors market reached a value of US$ 1.7 Billion in 2023, and projected the 7MM to reach US$ 3.2 Billion by 2034, exhibiting a growth rate (CAGR) of 5.61% during the forecast period from 2024 to 2034.
Female Infertility Market: The 7 major female infertility market reached a value of US$ 2.0 Billion in 2023, and projected the 7MM to reach US$ 2.7 Billion by 2034, exhibiting a growth rate (CAGR) of 2.76%during the forecast period from 2024-2034.
Filariasis Market: The 7 major filariasis market is expected to exhibit a CAGR of 7.92% during the forecast period from 2024-2034.
Contact US
IMARC Group
134 N 4th St. Brooklyn, NY 11249, USA
Email: Sales@imarcgroup.com
Tel No:(D) +91 120 433 0800
Phone Number: - +1 631 791 1145, +91-120-433-0800