Food and Drug Administration (FDA)
NEWS
In addition to lowering of blood sugar, Mounjaro also enabled patients to lower their weight by 15 to 23 pounds. The drug compared well to other diabetes medications including Novo Nordisk’s semaglutide.
U.S. Food and Drug Administration(FDA) has granted Orphan Drug designations to Editas Medicine and Neurocrine Biosciences.
The U.S. Food and Drug Administration (FDA) has approved Mitsubishi Tanabe Pharma America’s oral alternative to its own treatment for amyotrophic lateral sclerosis (ALS).
BridgeBio said it would sell the voucher for $110 million but did not disclose the identity of the company planning to acquire the voucher.
The rejection appears to be related to pre-approval inspection issues that need to be resolved before it can be approved.
Marinus shared positive news in its financial report for the first quarter, led by updates from its Phase III trial on a drug for RSE and research efforts for rare diseases.
Stealth BioTherapeutics has received Orphan Drug Designation from the U.S. Food and Drug Administration (FDA) for elamipretide.
TCR² Therapeutics provided updates regarding the 2022 first quarter financial results and progress made towards regulatory approval for gavo-cel and TC-510.
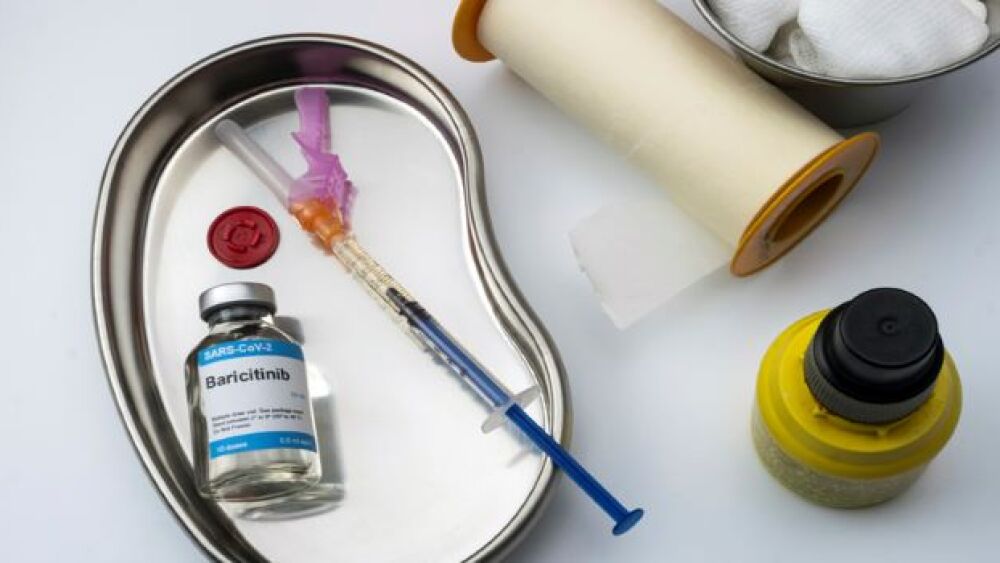
The FDA has given approval for the commercialization of Eli Lilly and Company and Incyte’s Olumiant, setting a precedent for upcoming COVID-19 treatments.
JOBS
IN THE PRESS