Food and Drug Administration (FDA)
NEWS
Mirati Therapeutics’ adagrasib is going up against Amgen’s Lumakras in the KRAS race, and according to Friday pre-market trading, the results shared Thursday don’t seem to be enough for investors.
Two researchers affiliated with Northwestern University have discovered a new drug that may outperform existing treatments for amyotrophic lateral sclerosis (ALS).
Pfizer announced the FDA has granted its combinatorial therapeutic ervogastat/clesacostat Fast Track Designation that is intended for the treatment of NASH with liver fibrosis.
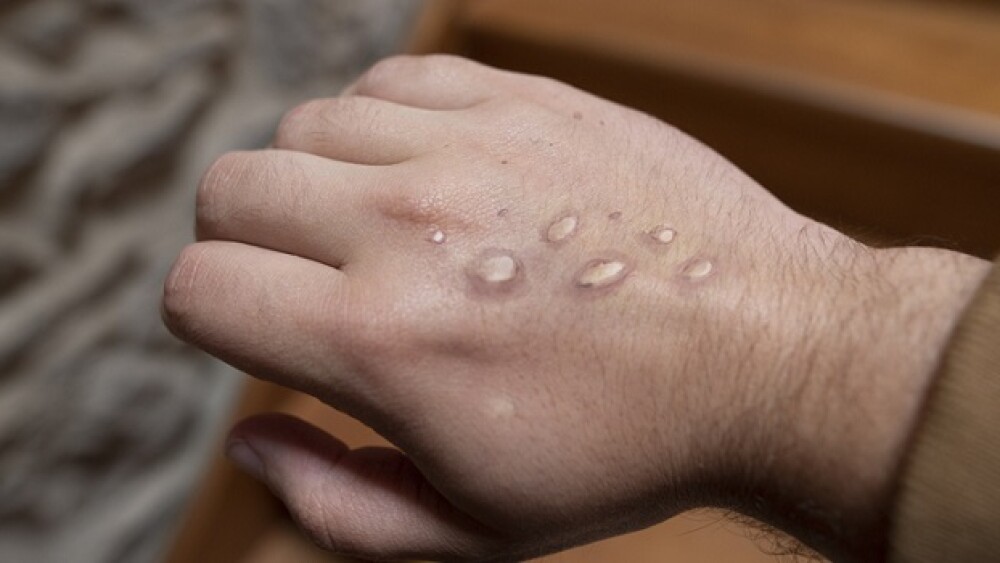
Warnings of Monkeypox went unheeded as scientists around the world began to uncover clues about the disease’s origin. Here are the latest updates on the monkeypox virus.
The FDA’s Vaccines and Related Biological Products Advisory Committee is set to review clinical data on June 7 and potentially suggest approval of NVX-CoV2373.
Antios Therapeutics received a clinical hold on its Hepatitis B virus (HBV) combinatorial therapy from the U.S. Food and Drug Administration on Wednesday.
Tyvaso DPI marks the first approval of a dry powder inhaler for the treatment of pulmonary arterial hypertension and pulmonary hypertension associated with interstitial lung disease.
Concerns are mounting for people that have been given Pfizer’s Paxlovid treatment regimen after contracting COVID-19, only to become positive for the infection once again.
In the third and most recent FDA letter, Verrica noted that none of the issues were specific to VP-102 and how it is manufactured.
JOBS
IN THE PRESS