Invivyd
NEWS
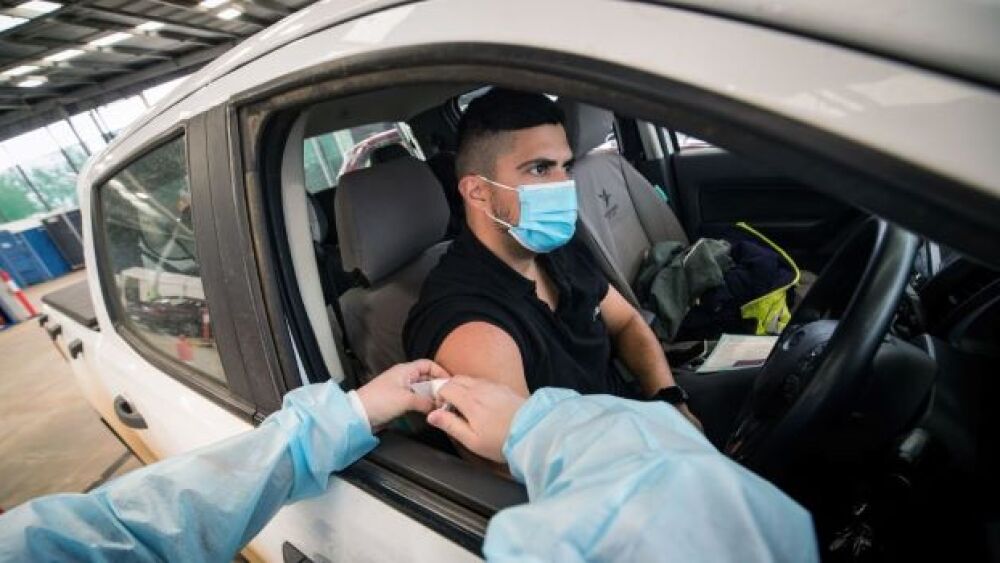
The regulator has allowed for emergency use of Invivyd’s monoclonal antibody Pemgarda as a COVID-19 pre-exposure prophylaxis for moderately or severely immunocompromised patients.
Adagio, Aravive, PolyPid, Harpoon, Durect, Nitrase, Kallyope, TScan and many more made major leadership decisions this week.
It was a particularly busy week for clinical trial announcements. Let’s take a look.
Although it looks that we’re on the downside of the COVID-19 pandemic in the U.S., biopharma companies are still working on developing better treatments and preventions.
Adagio Therapeutics has appointed current COO David Hering as interim CEO, replacing Tillman Gerngross, Ph.D., who communicated his intention to resign in mid-February.
Weeks after announcing that its lead monoclonal antibody was demonstrating efficacy against the Omicron variant, Adagio Therapeutics CEO Tillman Gerngross steps down.
Amagma Therapeutics announced a licensing agreement with Innovent Biologics for up to three enzyme specific inhibitors derived from Amagma’s proprietary SEIZMIC Platform.
Adagio cited three recent independent, in vitro studies showing that ADG20, which is currently undergoing Phase II/III clinical trials, has neutralization activity against Omicron.
The year kicked off with a bang as multiple companies raced to a public listing.
JOBS
IN THE PRESS