Akero Therapeutics
NEWS
Novo will add Akero’s efruxifermin to its MASH portfolio, which includes Wegovy after the GLP-1 gained an FDA nod in the indication earlier this year.
Akero Therapeutics, 89bio, Boston Pharmaceuticals and more are working to bring novel treatment options for metabolic dysfunction-associated steatohepatitis to a market that could reach $16 billion by 2033.
While Madrigal Pharmaceuticals secured the first FDA drug approval for metabolic dysfunction–associated steatohepatitis, Akero Therapeutics is developing what may serve as a viable alternative treatment for precirrhotic disease.
New 96-week data show Akero Therapeutics’ efruxifermin can improve fibrosis by at least one stage without metabolic dysfunction-associated steatohepatitis worsening in more patients versus placebo.
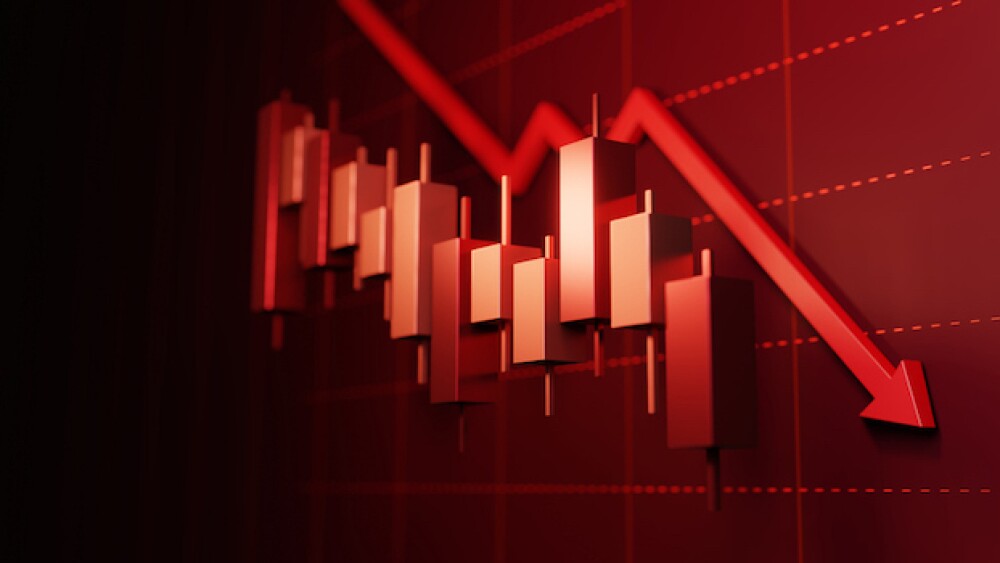
Shares of Akero Therapeutics took a hit after the company missed the primary endpoint in a Phase IIb study of efruxifermin in nonalcoholic steatohepatitis.
Both low and high doses of Akero Therapeutics’ lead candidate efruxifermin failed to significantly outperform placebo at improving liver fibrosis without worsening non-alcoholic steatohepatitis.
Non-alcoholic steatohepatitis patients treated with the company’s efruxifermin saw significant improvements in liver fat and biomarkers of liver damage, fibrosis and cardiometabolic health.
Industry leaders say the second generation of interventions for nonalcoholic steatohepatitis is likely to succeed where first-generation approaches stumbled.
Oramed announced positive mid-stage data that showed its investigational drug ORMD-0801 reduced liver fat content in type 2 diabetes patients who have been diagnosed with NASH.
JOBS
IN THE PRESS