All News
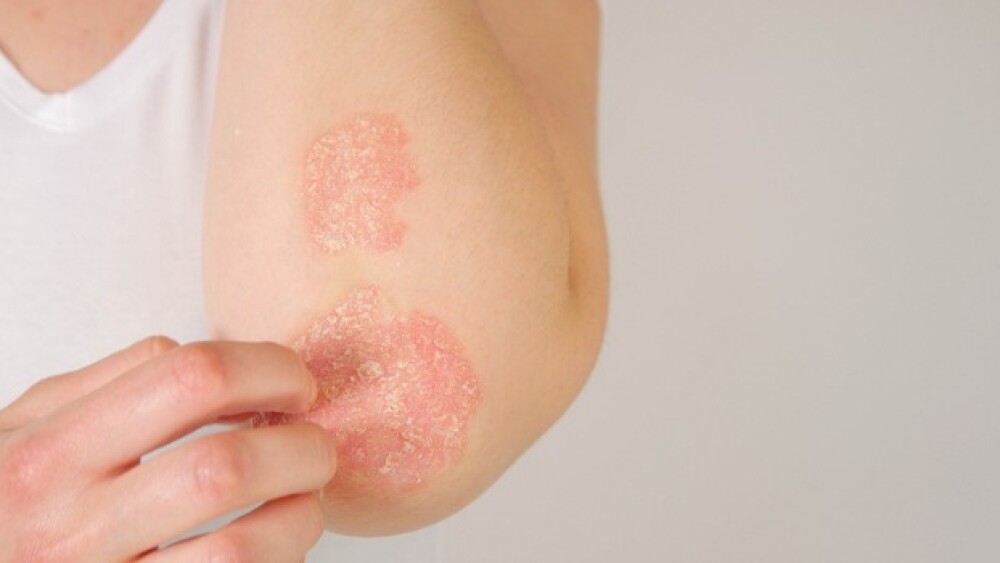
Fresh off its $259 million Series C funding round, Alumis unveiled mid-stage data Saturday for its TYK2 inhibitor ESK-001 demonstrating strong symptomatic improvement in moderate-to-severe plaque psoriasis.
Johnson & Johnson’s Protagonist-partnered oral psoriasis candidate was able to sustain its therapeutic benefit through one year, according to data presented on Saturday at the American Academy of Dermatology annual meeting.
Novo Nordisk’s blockbuster weight-loss drug Wegovy was approved on Friday by the FDA to reduce the risk of cardiovascular death, heart attack and stroke in adults who have cardiovascular disease and are obese or overweight.
In this deep dive, BioSpace examines what’s next for Leqembi, the true cost of anti-amyloid antibodies, and what other Alzheimer’s treatments are coming down the pipeline.
The approval, which Bristol Myers Squibb reported on Thursday, positions the company to compete with Astellas and Pfizer’s Padcev.
According to Fresenius Kabi, Tyenne is the first biosimilar to Genentech’s Acterma which has both IV and subcutaneous formulations approved by the FDA.
The company announced Thursday it terminated its BTK inhibitor evobrutinib for multiple sclerosis, which did not meet the primary endpoints in two late-stage trials.
MindMed’s stock price jumped over 50% Thursday as its LSD-based candidate for generalized anxiety disorder got a breakthrough therapy designation from the FDA.
President Biden called for expanding the Inflation Reduction Act to lower prescription drug prices. What would Trump do if he wins the 2024 election?
BMS and J&J will meet with the Oncologic Drugs Advisory Committee Friday to discuss their CAR-T therapies Abecma and Carvykti as the companies seek their approval as earlier lines of treatment.
The FDA will convene its Peripheral and Central Nervous System Drugs Advisory Committee to discuss Eli Lilly’s application for its Alzheimer’s disease antibody donanemab, the company announced Friday.
Friday’s topline data from the Phase III PHOENIX trial of Relyvrio, which won approval in 2022, showed no significant difference on either the primary or secondary endpoints, according to Amylyx.
AstraZeneca and Sanofi’s respiratory syncytial virus immunizing antibody Beyfortus reached effectiveness of 90% against infection-related hospitalization in infants, according to the Centers for Disease Control and Prevention.
Judge Zahid Quraishi on Thursday appeared unconvinced by the pharmaceutical companies’ arguments that the Inflation Reduction Act’s Drug Price Negotiation Program violated the U.S. Constitution.
BeiGene’s Brukinsa becomes the first BTK inhibitor approved for follicular lymphoma, the most common type of low-grade non-Hodgkin lymphoma.
Gilead Sciences is partnering with Dutch biotech Merus to find novel dual tumor-associated antigens that target tri-specific antibodies.
The antibody-drug conjugate beat the standard of care in second-line multiple myeloma, the company reported on Thursday, as it seeks to get the cancer drug back on the U.S. market.
The FDA’s busy week ahead involves three decision dates for potential industry firsts and a highly anticipated advisory committee meeting for two CAR-T therapies.
Novo Nordisk’s early-stage amylin and GLP-1 co-agonist elicited a 13.1% reduction in body weight, with an overall favorable safety profile, the Danish drugmaker reported Thursday at an investor event.
The Anglo-Swedish drugmaker is making the investment to bolster its vaccine manufacturing and research and development capabilities in the U.K.