All News
The company has several targeted therapeutics in early-stage trials and preclinical development for cancer types with high expression levels of the protein.
The lawsuit, brought by Arbutus Biopharma, marks the latest legal action in an ongoing battle for intellectual property underlying mRNA vaccines for COVID-19.
Liver toxicities triggered a partial clinical hold on Merck KGaA’s trial studying its BTK inhibitor candidate for multiple sclerosis.
A look at the differences among types of retirement accounts, and how to evaluate which is best for you
Junshi Biosciences announced Tuesday that its ovarian cancer candidate, senaparib, met the primary endpoint in a Phase III interim analysis.
Following mixed Phase I/II data for its homocystinuria hopeful pegtarviliase, Aeglea lays off all but 10 employees and launches a sweeping strategic review.
An interim analysis of the Phase III P302 study showed Moderna’s investigational flu shot, mRNA-1010, fell short of the statistical threshold for early success.
A California judge denied Elizabeth Holmes’ motion for release pending appeal, ruling that the former Theranos CEO must serve her prison sentence while she appeals her guilty verdict.
BioSpace spoke with two professionals who have made the switch from tech roles to biotech to find out the benefits, challenges and tips for a smooth transition.
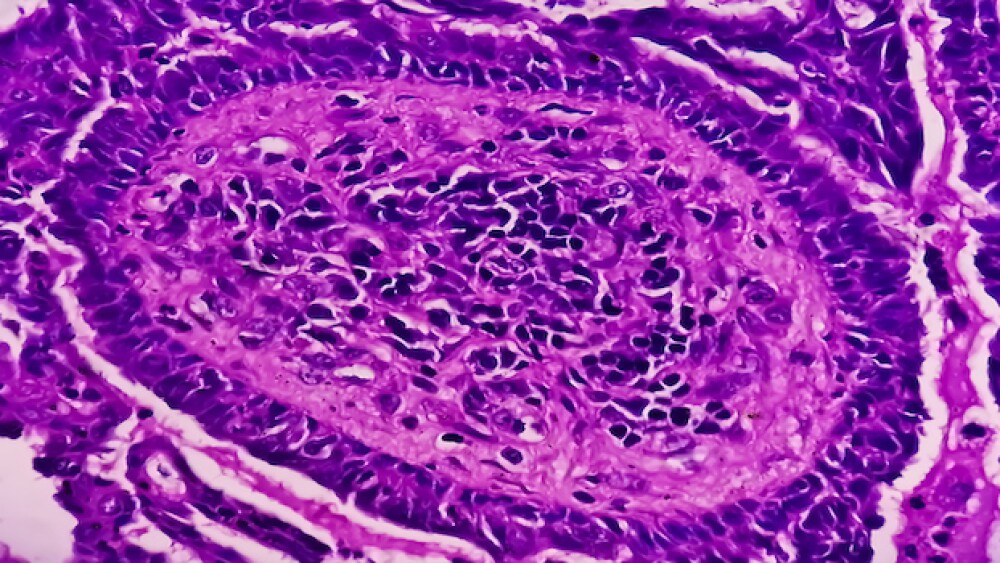
Data from two mid-stage studies show HI-Bio’s investigational therapy felzartamab lowers pathogenic antibody titers in patients with primary membranous nephropathy (PMN).
Karuna Therapeutics’ KarXT and Acadia Pharmaceuticals’ pimavanserin are sparking hope that a treatment for the negative symptoms of schizophrenia could be on the horizon.
In an open letter published Monday, more than 480 biopharma industry leaders expressed their support for the FDA after a Texas federal judge ruled to hold the approval of the abortion pill mifepristone.
The Boston Business Journal reported the layoffs would affect the multiple sclerosis team, but Biogen declined to confirm the details.
Choose your raw materials wisely
AbbVie and Johnson & Johnson will withdraw the accelerated approvals for Imbruvica in mantle cell lymphoma and marginal zone lymphoma, the companies announced Thursday.
The FDA is set to decide on a Humira biosimilar and hold an Adcomm meeting for an Alzheimer’s agitation treatment. Also on its calendar for April are decision dates for two vaccine hopefuls.
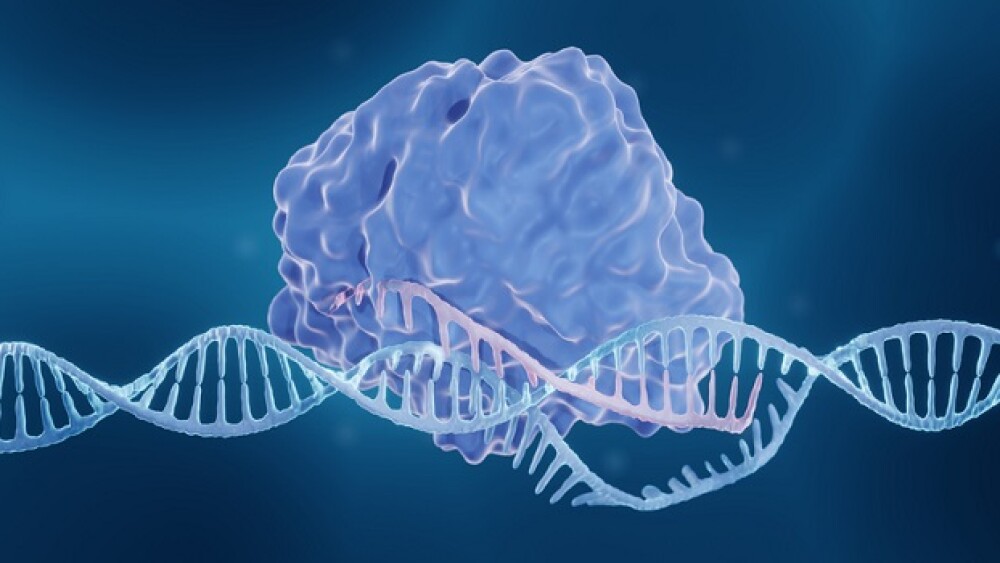
Currently, there are no gene editing–based treatments on the market, but the technology continues its march toward potential FDA approval, with several products in mid- and late-stage trials.
After 12 years on the market, the FDA announced it has withdrawn its previous approval for the only drug on the market to prevent preterm birth, Covis Pharma’s Makena.
Results from two late-stage trials showed the Merck-Eisai combo treatment failed to boost survival in two difficult-to-treat advanced cancers.
At the World Vaccine Congress, antimicrobial resistance as a global health crisis grabbed top billing.