All News
The new fund brings Pivotal bioVenture Partners’ total capital to $689 million, which the venture capital firm intends to pump into young biotechs developing “innovative and impactful therapeutics.”
There is no evidence that the lapses have harmed patients who received Spikevax or clinical trial participants who received investigational shots.
Following a U.S. appeals court decision, the company said it is preparing to divest GRAIL through a third-party sale or capital markets transaction.
The biotech reported topline data from a mid-stage study of MM-120, its LSD-based drug, in patients with generalized anxiety disorder.
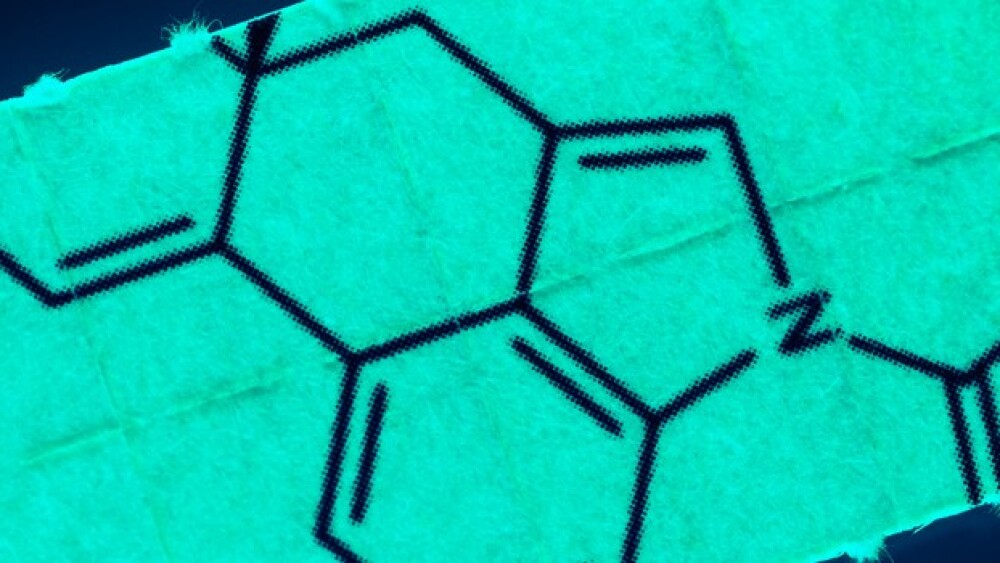
A European Medicine Agency panel on Friday reaffirmed its decision not to renew the conditional marketing authorization for GSK’s multiple myeloma drug Blenrep.
President Joe Biden has long promised to stand up to Big Pharma, lower prescription drug prices and limit the power of drugmakers—a pledge he seems intent on keeping.
Merck’s HIF-2α inhibitor Welireg has been approved by the FDA to treat advanced renal cell carcinoma after treatment with PD-1 or PD-L1 and VEGF-TKI-based therapies.
Despite pricing concerns for bluebird bio’s FDA-approved sickle cell disease gene therapy, the biotech has inked a deal with an unnamed commercial payer “representing approximately 100 million covered lives” in the U.S.
A week after securing FDA approval, a European Medicines Agency committee has endorsed Vertex and CRISPR Therapeutics’ Casgevy for sickle cell disease and transfusion‑dependent beta thalassemia.
Amid falling COVID-19 revenues, Pfizer continues to invest in antibody-drug conjugates in a deal with Nona Biosciences worth $1 billion as its $43 billion Seagen buy closed on Thursday.
The regulator placed the clinical hold on the Chinese biotech’s trio of CAR-T cell therapy candidates after an inspection of its Durham, North Carolina manufacturing facility.
Despite the lack of a randomized clinical trial to support eflornithine’s efficacy, the regulator approved US WorldMeds’ oral maintenance treatment for high-risk neuroblastoma in adults and children.
At the urging of the Biden administration, Sanofi and AstraZeneca are pledging 230,000 additional doses of their respiratory syncytial virus infant immunization Beyfortus in January 2024.
The combination of Keytruda and an mRNA vaccine reduced the risk of death or relapse in patients with the most deadly form of skin cancer after three years in a Phase IIb study.
The Biden administration on Thursday said 48 drugs covered under Medicare’s Part B may be subject to inflation rebates in the first quarter of 2024 under the Inflation Reduction Act.
What needs to happen for funding in biopharma to bounce back? BioSpace’s Lori Ellis discusses the macroeconomic environment and biopharma funding outlook with venture capital guests Ansbert Gadicke, Martin Gershon and Mike Goguen.
The U.S. Supreme Court has agreed to the Biden administration’s request to review lower-court rulings that would curtail access to the abortion pill mifepristone, with a decision expected in the summer.
After a “reasonably strong year” in 2023, next year will see “similar levels” of M&A activity with increased investor interest in weight loss and cardiovascular diseases, contends professional services firm PwC.
If approved by the FDA, the MDMA-assisted treatment for individuals with post-traumatic stress disorder would be the first U.S. psychedelic-assisted therapy.