All News
BioNTech will pay $50 million in cash and purchase $200 million of Autolus Therapeutics’ shares to progress the companies’ respective CAR-T candidates to commercialization.
On Thursday, Kyverna Therapeutics is debuting on the Nasdaq with an upsized initial public offering which the biotech will use to support its pipeline of anti-CD19 CAR T therapy candidates.
Thursday’s full-year financial results from AstraZeneca showed a revenue crash of more than 90% for COVID-19 medicines, while cancer, cardiovascular and rare diseases all generated double-digit revenue growth.
The European Medicines Agency is looking into how Novo Holdings’ recently announced $16.5 billion acquisition of contract manufacturer Catalent might affect the availability of drugs.
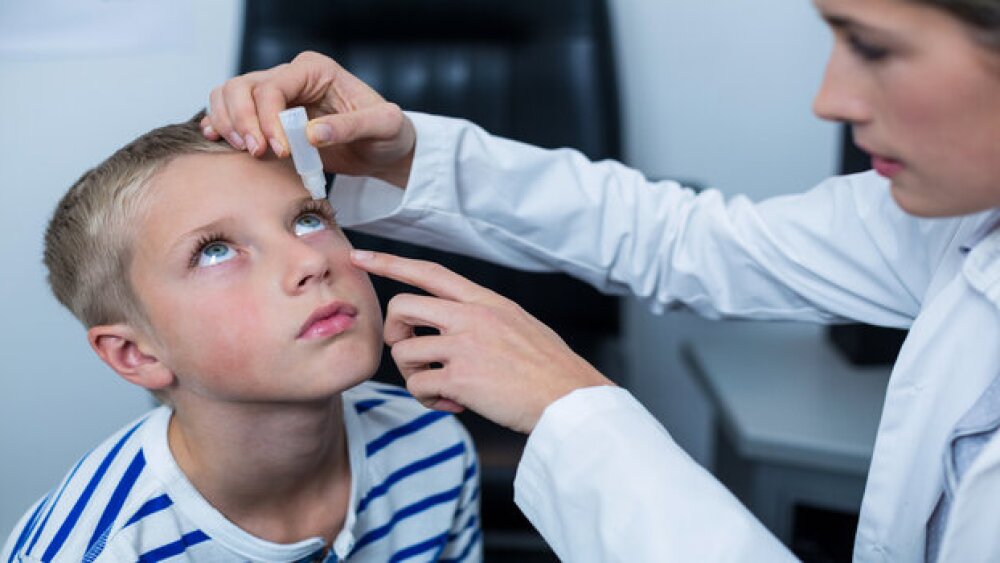
Under the FDA’s compassionate use program, an eyedrop formulation of Krystal Biotech’s Vyjuvek restored the vision of a teenager with the rare genetic disease dystrophic epidermolysis bullosa.
A proposed class-action lawsuit accuses Johnson & Johnson of failing to negotiate fair prescription drug prices for its employees, but experts note that the company is also on the hook for benefits costs.
Takeda, AbbVie and Moderna are among the companies with open positions.
Jazz Pharmaceuticals is acquiring Redx Pharma’s KRAS inhibitor program including preclinical-stage drug candidates, with the companies working to advance assets through IND-enabling studies.
This week Lori, Greg and Tyler discuss drug pricing reforms. CMS sent offers to manufacturers of the 10 drugs that have been selected for Medicare price negotiations. What’s the best way forward that benefits patients while still supporting the innovation that makes these drugs possible? How will the election impact negotia
While analysts are bullish on Novo Holdings’ $16.5 billion acquisition of Catalent, they say it raises questions for companies that have contracted the CDMO for manufacturing.
Venture capital firm Scion Life Sciences, led by investors Samuel Hall, Aaron Kantoff and Tadd Wessel, seeks to establish biotechs through to maturity as late- or commercial-stage companies.
Eli Lilly on Tuesday said it is already making manufacturing investments for orforglipron, its next-generation oral weight-loss candidate that recently moved into Phase III development.
Hit with lower sales of its COVID-19 antiviral Veklury and the weak performance of its HIV franchise, Gilead Sciences reported a 4% year-over-year revenue loss in the fourth quarter of 2023.
Thanks to a rough launch of its Biogen-partnered Alzheimer’s disease treatment, Eisai will likely miss its target of treating 10,000 patients with Leqembi by the end of March 2024.
With manufacturing issues persisting, last year’s shortages of medicines, including chemotherapies, weight-loss drugs and antibiotics, will continue into 2024.
Politics aside, both the government and the pharmaceutical industry want to bring affordable effective therapies to patients. Implementation is the obstacle. Working together is the only way to modify the IRA to do what it intends to do: benefit patients.
The monoclonal antibody Nipocalimab, which J&J acquired from Momenta Therapeutics, met its primary endpoints in mid-stage and late-stage trials in myasthenia gravis and Sjögren’s disease.
The French vaccine maker on Monday said it sold a priority review voucher, which was awarded by the FDA in November 2023 alongside an approval for its chikungunya vaccine, to an undisclosed buyer.
Design patents, trademarks, copyright and trade secrets offer lower cost, and sometimes underappreciated, forms of intellectual property.
Eli Lilly on Tuesday reported more than $9.3 billion in revenue in the fourth quarter of 2023, beating Wall Street expectations, thanks to demand for its weight-loss drug Zepbound and diabetes treatment Mounjaro.