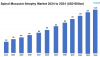
Spinal muscular atrophy
New data shows Zolgensma, Novartis’ gene therapy for spinal muscular atrophy, has the potential to be used presymptomatically in juveniles.
After discovering promising indicators on the path to the development of Branaplam (LMIO70) for spinal muscular atrophy, Novartis now hopes to repurpose the drug for the treatment of Hungtington’s disease.
Biogen recently announced plans to initiate a Phase IV clinical trial to determine the benefit of Spinraza in patients who received Zolgensma. The two therapies have markedly different ways of treating the disease.
Genentech, a Roche company, announced the U.S. Food and Drug Administration (FDA) had approved its Evrysdi (risdiplam) for spinal muscular atrophy (SMA) in adults and children two months of age and older.
Biogen announced new data from the NURTURE trial of pre-symptomatic patients with spinal muscular atrophy (SMA).
Genentech, a Roche company, presented one-year data from FIREFISH Part 2, its pivotal trial of risdiplam in infants one to seven months old with symptomatic Type 1 spinal muscular atrophy.
Genentech’s Risdiplam showed significant improvement in motor function in people aged 2-25 who have been diagnosed with Type 2 or 3 spinal muscular atrophy.
Genentech announced positive topline results from its pivotal Part 2 of the FIREFISH study looking at risdiplam in infants aged 1-7 months with Type 1 SMA.
The company has suggested that it plans to undercut both Biogen and Novartis on price in order to make up for being third-to-market.
Filing submission includes 12-month data from pivotal FIREFISH and SUNFISH trials in a broad population of people living with Types 1, 2 or 3 SMA
PRESS RELEASES