TAMPA, Fla., April 28, 2014 /PRNewswire-USNewswire/ -- ISPE, the International Society for Pharmaceutical Engineering, announced today that it will work with stakeholders world-wide to produce a Drug Shortages Prevention Plan to guide the pharmaceutical and biopharmaceutical industry in establishing reliable, robust and resilient supply chains that provide quality medicines to patients without interruption. The Plan, which will be based on ISPE research with the input of its membership, company leaders and regulators, will serve as a roadmap that when implemented, can significantly reduce drug shortages. The Plan will address optimal organizational strategies, such as aligned governance and communication practices, effective manufacturing and quality systems, and appropriate measures of supply chain robustness and quality. This effort addresses rising concerns around drug shortages within companies, among global health authorities, and for patients who depend on a reliable and available supply of quality medicines.
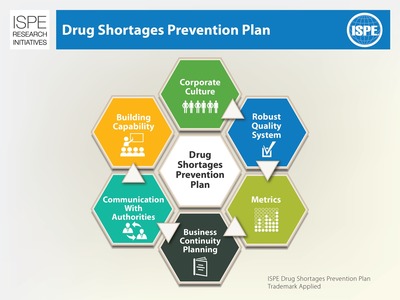
The ISPE Drug Shortages Prevention Plan will be the Society's second major output on this topic since launching its Drug Shortages Initiative in 2012. This second phase of the Initiative is aimed at addressing the root causes of drug shortages to prevent delay of supply. The 2013 survey provided clear evidence that mitigating shortages requires a holistic approach that encompasses both the organizational and technical issues affecting drug manufacturing and quality. "How much of the increasing number and severity of incidents of drug shortages within our industry are fundamentally tied to a lack of understanding, or even concern for, the risk profiles of our current supply chain structure?" commented Andy Skibo, Regional VP, Biologics Supply, AstraZeneca/MedImmune. "I therefore welcome ISPE's initiative to tackle the root causes of shortages for the benefit of all stakeholdersISPE Members, industry companies, regulators and health authorities, third-party providers and patients."
The ISPE Drug Shortages Prevention Plan will provide a key component of the Society's input to a European multi-association task force, moderated by ISPE, which intends to provide EMA a proposal and plan that address the prevention of drug shortages due to manufacturing quality issues. "I'm delighted to be collaborating with colleagues from the Parenteral Drug Association to deliver our proposal to the EMA later this year, and appreciate the support we are receiving from EFPIA, EGA, AESGP and PPTA as well as regulators representing the EMA, the UK, Irish, Spanish and French national agencies," said Dr. John Berridge, ISPE's Advisor and inter-Association task force moderator.
In 2012, ISPE formed a Drug Shortage Task Force in order to help stakeholders better understand the root causes of global drug shortages and to define mitigation strategies that can help prevent drug shortages. In ISPE's initial approach to this challenge, the group led a comprehensive survey in 2013 that revealed multi-factorial causes of drug shortages, as well as success strategies for avoiding supply interruptions. Those companies that have adopted organizational drug shortage prevention strategies, including contemporary governance, progressive cultures and effective quality systems, were successful in the avoidance of drug shortages and supply interruptions. "Our research findings indicated that, while shortages are often the result of quality systems or related deficiencies, what was equally profound was that those companies with dedicated and sophisticated systems for avoiding drug shortages were clearly more successful in avoiding supply disruptions and shortages," said Nancy Berg, ISPE's President and CEO. "Recognizing the impact of these success strategies motivated ISPE to develop its Drug Shortages Prevention Plan and to develop guidance on organizational strategies for drug shortages prevention, which include the engagement of company leadership and execution of governance, culture, especially a culture of quality risk management throughout the supply chain, and insightful quality metrics."
ISPE's Drug Shortages Prevention Plan will feature an organization and product lifecycle approach to ensuring a more reliable supply of medicines through development of company-wide drug shortages strategic plans, aligned governance, good communication, ongoing capability building (which includes training), proactive regulatory interaction and integrated quality systems. The development of industry-wide quality metrics is being addressed by the Society through a parallel Quality Metrics Initiative, led by another ISPE expert team recommending to FDA and other health authorities a set of common metrics on which the health and reliability of a company's production and quality systems and products can be assessed.
For more information on ISPE's Drug Shortages Prevention Plan or other ISPE Initiatives, contact Ms. Carol Winfield, Director of Regulatory Operations, at cwinfield@ispe.org.
About ISPE
ISPE, the International Society for Pharmaceutical Engineering, is the world's largest not-for-profit association serving its Members through leading scientific, technical and regulatory advancement throughout the pharmaceutical lifecycle. The 20,000 Members of ISPE are building solutions in the development and manufacture of safe and effective pharmaceutical and biologic medicines and medical delivery devices in more than 90 countries around the world. Founded in 1980, ISPE has its worldwide headquarters in Tampa, Florida, USA. Visit www.ISPE.org for more information.
For more information contact:
Danielle Hould
ISPE Manager, Marketing Communications
Tel: +1-813-960-2105, ext. 277
Email: dhould@ispe.org
www.ISPE.org

Photo: http://photos.prnewswire.com/prnh/20140428/81206
Logo - http://photos.prnewswire.com/prnh/20120912/DC73253LOGO
SOURCE ISPE
Help employers find you! Check out all the jobs and post your resume.
