Novartis
NEWS
It was, as usual, a pretty busy week in clinical trial news. Here’s a look.
Over the course of the past year, Novartis has seen significant success with the approval of five drugs that have blockbuster potential and the company sees an additional 25 potential blockbuster drugs in its pipeline.
“We are excited about entering into an agreement to acquire The Medicines Company as inclisiran is a potentially transformational medicine that reimagines the treatment of atherosclerotic heart disease and familial hypercholesterolemia,” said Vas Narasimhan, chief executive officer of Novartis.
Pharma Integrates 2019, which took place on 18th and 19th November, saw over 450 delegates from biotech, pharma, CDMOs and the supply chain come together to debate and share insights on key topics currently affecting the industry.
As usual, it was a busy week for clinical trial updates. Here’s a look.
Three years after Novartis opened a $1 billion research & development facility in Shanghai, the company is shifting the focus of the site from drug discovery to commercial development.
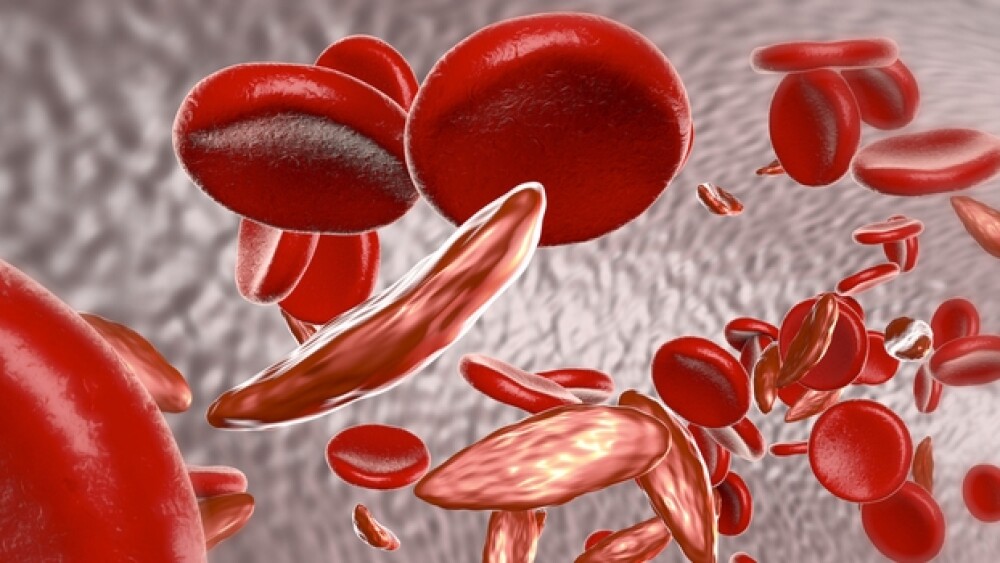
“The approval of Adakveo marks a new era in the treatment of sickle cell disease, a genetic condition that places an extraordinary burden of unpredictable pain crises on patients and their families,” said Susanne Schaffert, president of Novartis Oncology.
For BioSpace’s recent Ideal Employer report, life sciences professionals weighed in on the topic.
The newly-launched index takes a focused look at the breadth and depth of novel agents currently being developed within the most innovative pharmaceutical pipelines.
JOBS
IN THE PRESS