bluebird bio
NEWS
After a series of milestone approvals in the first half of 2023, the FDA is slated to decide on four more firsts before the year’s end.
The regulatory filing puts the company alongside Vertex and CRISPR, which also await FDA approval for their SCD gene therapy.
In an earnings call Wednesday, bluebird bio revealed it is unlikely to meet its first-quarter goal to submit a Biologics License Application for sickle cell disease (SCD) gene therapy lovo-cel.
bluebird bio, Inc. is expecting gross proceeds of $120 million with its newly announced public offering of 20,000,000 shares.
The FDA’s top five approvals in 2022 represent an eclectic mix of cancer, cardiovascular and rare disease drugs.
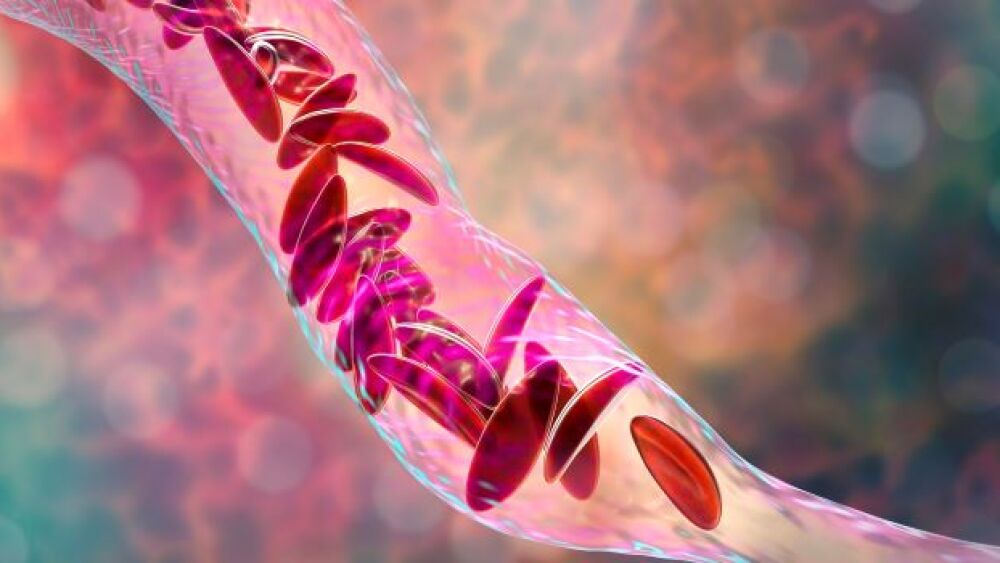
The FDA lifted its partial clinical hold on bluebird’s experimental sickle cell disease gene therapy for patients younger than 18.
While worthy advances have recently been made in sickle cell disease, companies such as Graphite Bio, bluebird bio, Vertex Pharmaceuticals and Editas Medicine have loftier ambitions.
The U.S. FDA had a busy week with a range of drug approvals, advisory committee meetings and classification announcements.
The FDA is heading into fall with a few Prescription Drug User Fee Act (PDUFA) dates. Here’s a look at this week’s upcoming action.
JOBS