Bayer
NEWS
Robert LaCaze is taking over the reins of Mnemo Therapeutics with the goal of overcoming key challenges in CAR-T therapies and driving the company’s lead assets into the clinic.
Clinical Catch-Up for February 21
Bayer is heading back to the FDA to seek approval for a new indication of its prostate cancer drug Nubeqa, following positive Phase III results.
As the market seems to be taking a turn against biotech stock, more companies are opting for private funding instead of going public for the moment. And VC firms are happy to jump in.
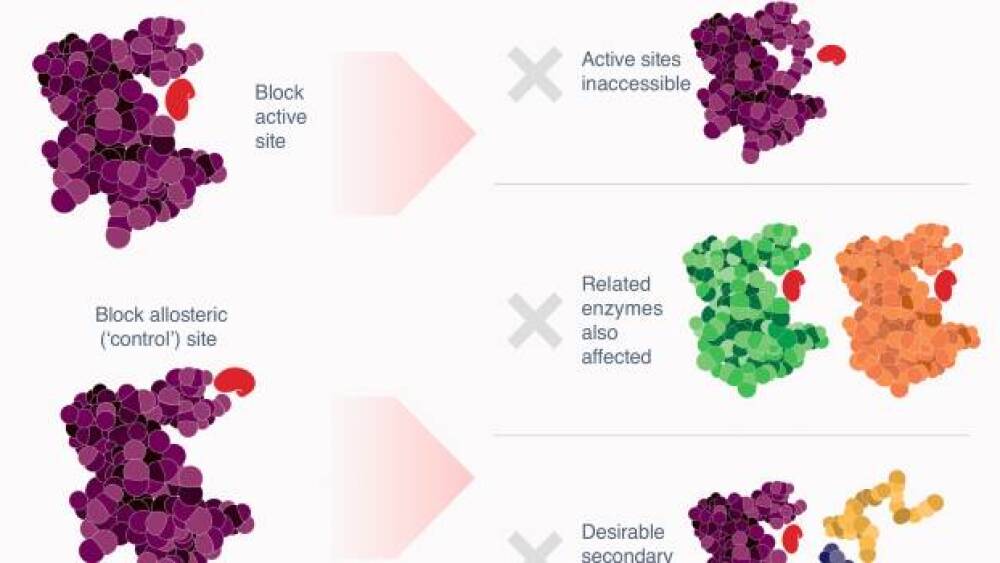
Exo Therapeutics’ approach is not only different, it has revolutionary potential.
Bayer joined in the $50 million Series A for San Francisco’s Indapta Therapeutics.
WuXi Biologics was amongst those on the list, with the U.S. stating that it was unable to verify the legitimacy and reliability of the company in its use of U.S. exports.
Bayer isn’t the only company to announce changes to its leadership team this morning. Editas, Biogen, Takeda, Merck and Vedere Bio also announced leadership changes.
After reviewing available data, Bayer decided to abandon its Phase II development of eliapixant as the benefit-risk profile was not worthwhile.
JOBS
IN THE PRESS