89bio
NEWS
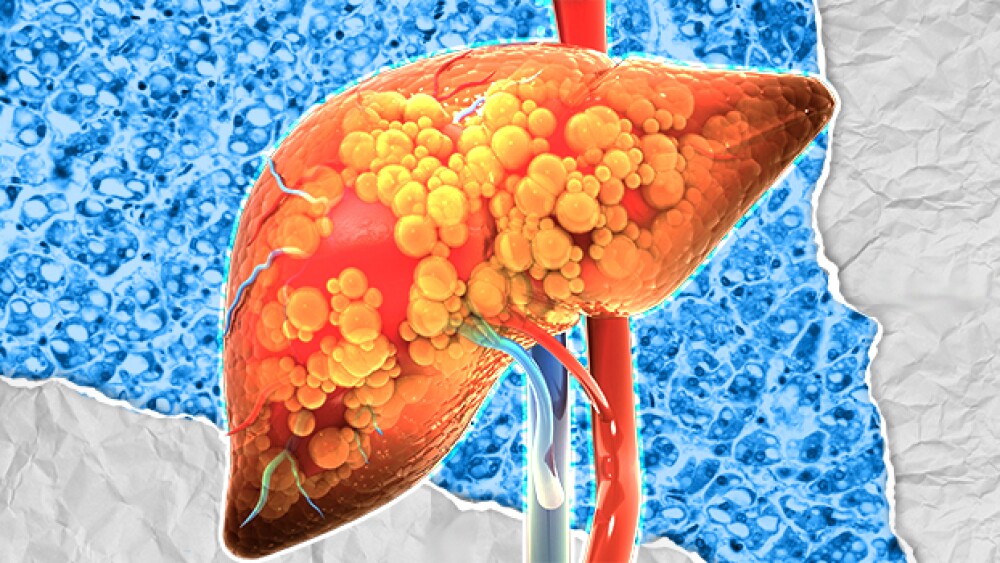
Akero Therapeutics, 89bio, Boston Pharmaceuticals and more are working to bring novel treatment options for metabolic dysfunction-associated steatohepatitis to a market that could reach $16 billion by 2033.
Madrigal Pharmaceuticals’ Rezdiffra (resmetirom) is the first-ever approved therapy for metabolic dysfunction-associated steatohepatitis—a decision experts say could signal a sea change in treatment of the disease.
Intercept’s failure to secure FDA approval for obeticholic acid (OCA) tablets in non-alcoholic steatohepatitis shines a light on safety challenges in the space, experts told BioSpace.
Data from the Phase IIb ENLIVEN trial showed 89bio’s pegozafermin met its primary histology endpoint in NASH patients, giving the company an edge in the competitive space.
89bio presented positive data for pegozafermin in SHTG, Tonix enrolled its first patient with Long COVID for TNX-102, Belite enrolled patients with STGD1 and more clinical trial news.
89bio reported positive topline results from ENTRIGUE Phase II trial of pegozafermin in patients with severe hypertriglyceridemia.
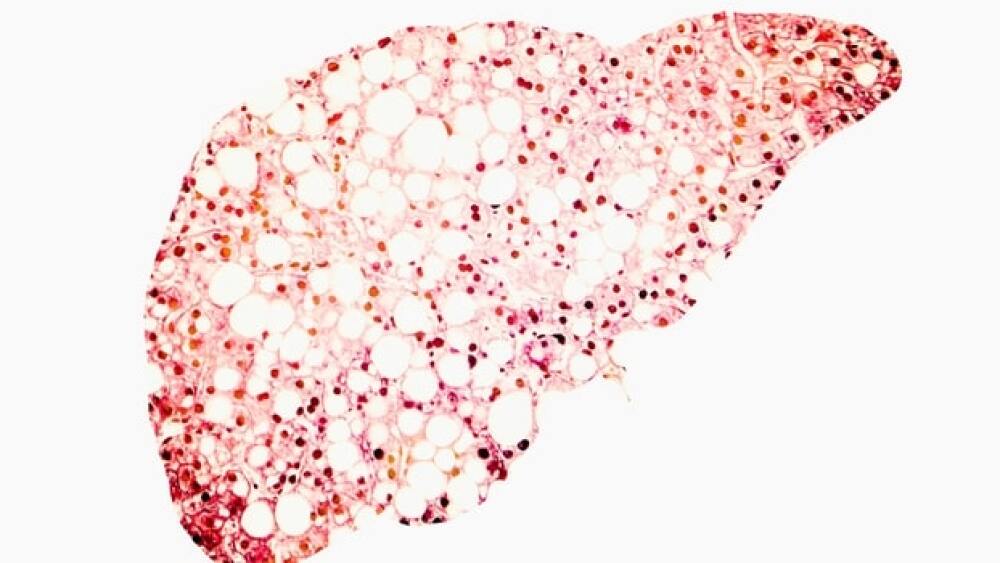
Shares of Madrigal Pharmaceuticals went up in premarket trading after the company announced positive topline data from its Phase III assessment of resmetirom in non-alcoholic fatty liver disease.
Sixty-three percent of patients met the primary endpoint of a 2-point or greater improvement in disease severity without worsening of fibrosis.
It was another busy week for clinical trial news. Here’s a look.
JOBS
IN THE PRESS