Approvals
AbbVie’s antibody-drug conjugate Elahere on Friday won the FDA’s full approval for the treatment of FRα-positive, platinum-resistant ovarian, fallopian tube and primary peritoneal cancers.
Duvyzat, which will be sold in the U.S. by ITF Therapeutics, joins a growing market that includes recently approved gene therapy Elevidys and corticosteroid Agamree.
Months after Johnson & Johnson turned its back on the hypertension treatment Tryvio, Idorsia has secured the FDA’s nod for the endothelin receptor blocker.
The recent FDA decision will likely mean more Medicare patients gain access to the blockbuster weight loss drug, experts say. Meanwhile, results continue to roll in for GLP-1 agonists for conditions beyond diabetes and obesity.
Takeda on Tuesday secured another label expansion for the kinase inhibitor, this time in the first-line setting for the treatment of Philadelphia chromosome-positive acute lymphoblastic leukemia.
Orchard Therapeutics on Monday secured the FDA’s first approval for an autologous gene therapy to treat the rare metabolic disease metachromatic leukodystrophy in children.
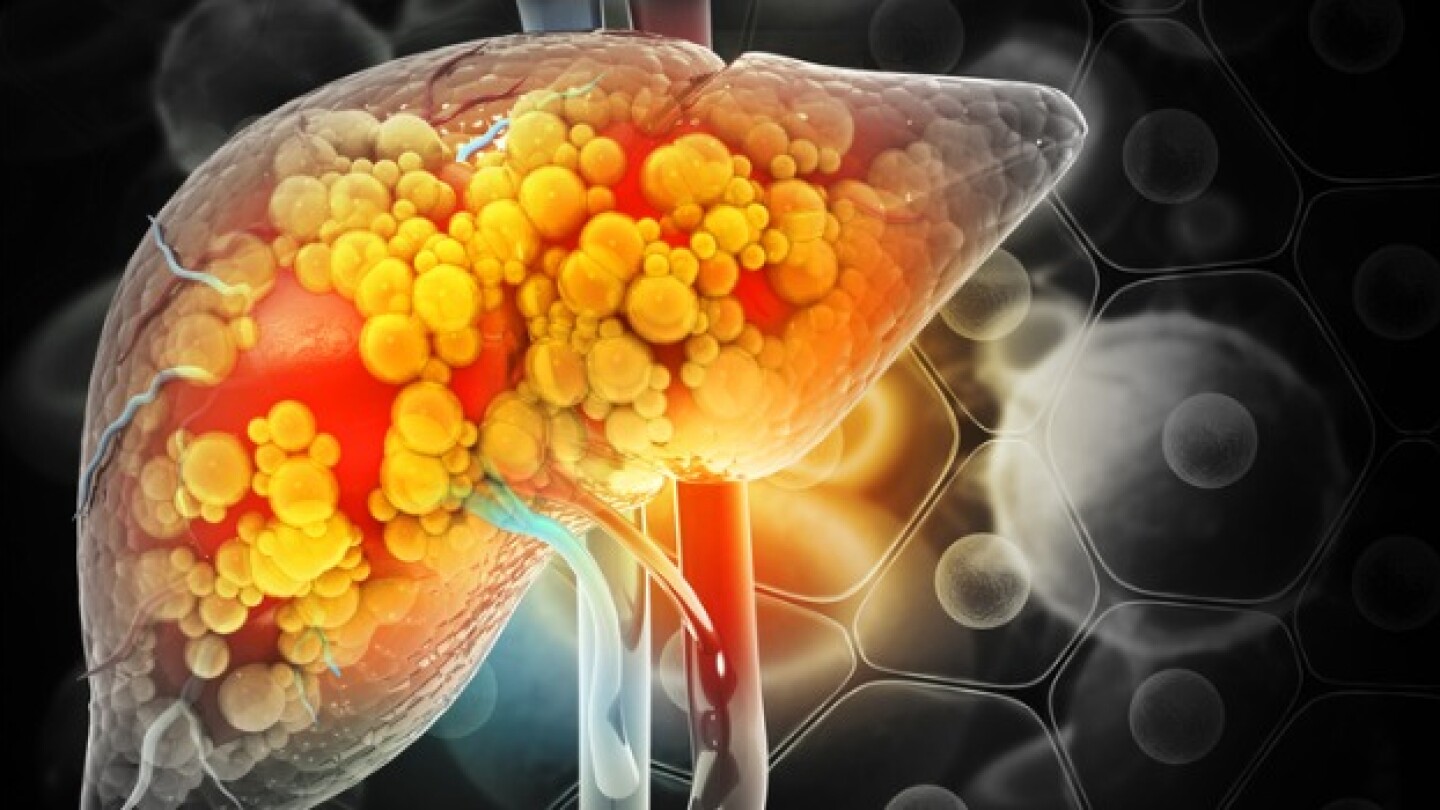
With its FDA approval last week and first-to-market advantage, Madrigal Pharmaceuticals’ Rezdiffra will set the standard for other metabolic dysfunction-associated steatohepatitis candidates in development.
The FDA approved Bristol Myers Squibb’s Breyanzi for chronic lymphocytic leukemia and small lymphocytic leukemia prior to Friday’s adcomm for the company’s other CAR-T therapy, Abecma.
After several delays, BeiGene on Thursday finally secured the FDA’s approval for its PD-1 inhibitor Tevimbra for the treatment of unresectable or metastatic esophageal squamous cell carcinoma.
If you’re confused by the NASH versus MASH indication, you’re not alone.
PRESS RELEASES