All News
In October, Vertex Pharmaceuticals announced stunning results in a type 1 diabetes patient who had been dosed with the company’s experimental therapy.
As the late-stage trials continue to progress, Talaris is rapidly ramping up its resources and investments.
The latest data from Merck and Ridgeback Biotherapeutics’ MOVe-OUT trial on the use of molnupiravir to treat COVID-19 in adults has shown a lower efficacy than previously reported.
The World Health Organization officially declared omicron – aka. B.1.1.529 – a variant of concern (VOC) on Friday.
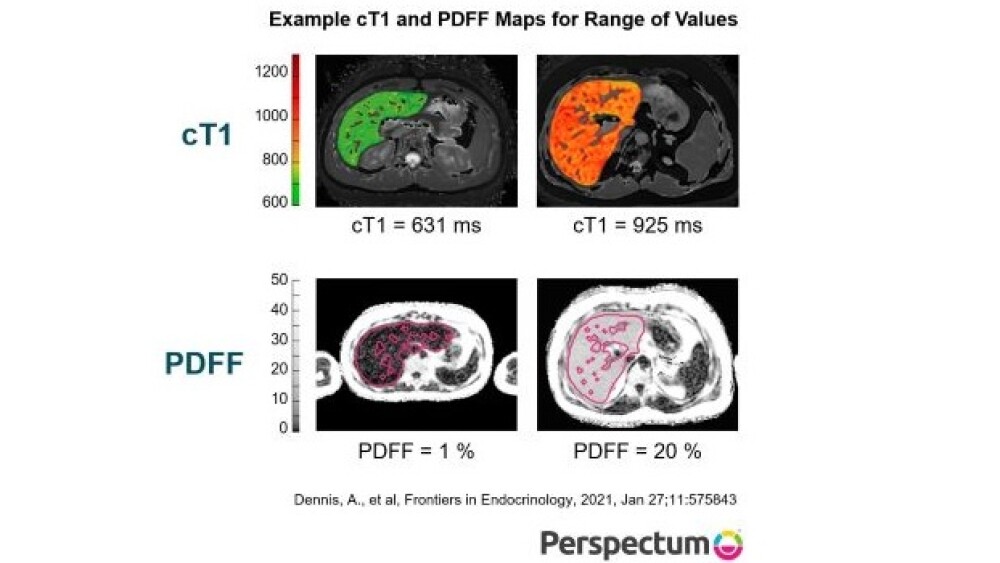
Treatment for non-alcoholic steatohepatitis (NASH), a severe form of fatty liver disease, consists mainly of lifestyle modification – specifically dieting and weight loss.
Thanksgiving week was marked by numerous clinical trial announcements. Here’s a look.
The U.S. Food and Drug Administration has a very busy calendar for the end of November and beginning of December. Here’s a look.
With the Thanksgiving holiday upon us, BioSpace felt it was important to give thanks for some of the positive things that have happened this year. And there are many!
Bonus BioGroup’s product is back in the news again as its cell therapy promises to treat late-stage COVID-19.
Both Gilead Sciences and Merck announced they were pausing a Phase II trial of islatravir and lenacapavir in HIV.
Biopharma and life sciences companies from across the globe provide updates on their businesses and pipelines.
Merck opted to license its second TriNKET immunotherapy candidate from Dragonfly Therapeutics. The option came one year after Merck licensed its first immunotherapy candidate.
A cardiovascular drug Pfizer licensed from Akcea Therapeutics in 2019 hit the mark in a Phase IIb dose-ranging study in patients with elevated non-HDL-C and triglycerides.
Compounds in these Peptides may be suitable for the treatment of cancers in which extracellular matrix metalloprotease (“MMP”) or other proteinases such as urokinase-type plasminogen activator (uPa) are implicated.
Although vaccines and effective therapeutics are powerful tools in preventing and treating COVID-19, we’re not done battling the disease just yet. For those stories and more, read on.
The precision medicine company is developing mutant selective and isoform-specific drugs in non-conventional ways for well-known targets in the oncology and autoimmune spaces.
Shares of Kura Oncology have plunged nearly 30% in premarket trading after the FDA placed a partial clinical hold on the company’s Phase Ib leukemia study following the report of a patient’s death.
The FDA approved Takeda’s Livtencity (maribavir) for adults and children 12 years or older with post-transplant cytomegalovirus (CMV) infection.
Plenty to be thankful for this week for these biopharma companies, especially with the influx of cash.
A preprint in bioRxiv from Penn State showed SARS-CoV-2 was detected in 33% (94) of the 283 white-tailed deer tested in Iowa between November 23, 2020 and January 10, 2021.