All News
Regeneron called it quits on an experimental osteoarthritis pain treatment that has raised safety flags and an experimental antibody for cat-allergic asthma.
Abeona Therapeutics will head to the FDA next year for the potential approval of its experimental recessive dystrophic epidermolysis bullosa (RDEB) therapy following positive Phase III data.
Emalex Biosciences closed a Series D funding round Thursday counting $250 million in earnings, much of which will bankroll a Phase III trial of ecopipam for Tourette syndrome.
Exelixis has inked a licensing agreement with Catalent to gain access to three of Catalent’s target programs with antibody and ADC candidates, the companies announced Thursday.
Moderna is dropping an IL-12 program in autoimmune disorders after AstraZeneca returned the rights, the mRNA leader announced Thursday in its third quarter filing.
After reaching a record high in 2021, venture capital dollars have tailed off in the biopharma industry in 2022. That said, a few biopharma hotbeds have still seen sizeable launch rounds.
Alkermes is exploring the potential spin-out of its cancer business into an independent, publicly traded company. Alkermes offered further details during an investor call Wednesday.
San Diego-based Arcturus Therapeutics signed a strategic collaboration and licensing deal with CSL Seqirus to help develop and commercialize vaccines.
Q1 is the time when many employers are actively recruiting new talent. Because it takes an average of 60 days to fill a job opening, Q4 might be the best time to apply for jobs in the life sciences.
Flush with cash from the growing success of its chemotherapy drug, Exelixis dropped $100M on two collaboration deals this week to invest in promising early clinical assets.
GSK scored a pivotal Priority Review from the FDA for its RSV vaccine candidate for older adults, which will expedite regulatory evaluation of its clinical data.
A Data Safety Monitoring Board overseeing the Phase Ib/II trial assessing UniQure’s gene therapy for Huntington’s disease recommended that enrollment in the higher-dose cohort could resume.
The future of Editas Medicine’s EDIT-101 will be determined later in November after a data readout is available. The data could inform the company whether there is a potential commercial path forward.
Surface Oncology announced it is pausing the development of its CD39-targeted antibody SRF617 and cutting around 20% of its workforce to focus on its lead anti-IL-27 program SRF388.
Lusaris Therapeutics launched with a $60 million Series A financing focus on advancing LSR-1019, a sublingual formulation of 5-MeO-DMT in development for TRD.
J.P. Morgan launched its new life science-focused private equity team, Life Sciences Private Capital, to support early- and growth stage biotech companies.
A new municipal law took effect Tuesday in New York City, requiring companies with four or more employees to include a specific pay range in every job posting.
Verge Genomics dosed its first patient in a Phase I trial studying VRG50635, while Stealth Bio’s SBT-272 was granted Orphan Drug designation by the FDA.
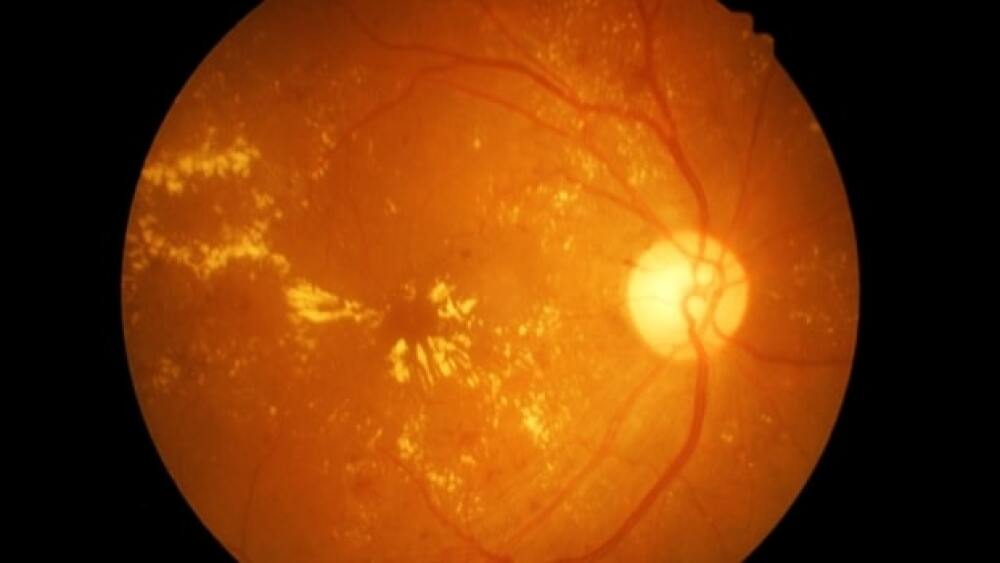
Unity announced advances in diabetic macular edema via Phase II study results, potentially allowing its recipients to return to tasks of daily living, like driving.
With $120 million in backing, HI-Bio launched two assets licensed from MorphoSys AG, including the anti-CD38 antibody felzartamab and HIB210, aimed at severe immune-mediated diseases.