All News
In its third acquisition this month, Eli Lilly is buying antibody-drug conjugates startup Emergence Therapeutics to bolster its cancer business.
After an initial rejection, BioMarin has finally secured the FDA’s approval for Roctavian, the first gene therapy in the U.S. for the most common form of the bleeding disorder.
The biopharma company instead chose to re-elect its existing board members.
The company Thursday reported positive topline results for a Phase III trial of its Alzheimer’s-related agitation treatment, while also disclosing improprieties by a principal investigator.
The initiatives coincide with an increase in the volume of data submitted to CDER and CBER—particularly in cell and gene therapy.
Eli Lilly announced Thursday it will acquire former collaborative partner Sigilon Therapeutics to deepen its diabetic foothold with a potentially functional cure for Type 1.
Of the 30 patients given CellTrans’ Lantidra in two studies, 21 were insulin-free for at least a year and 10 were insulin-free for more than five years.
Phase I/II data on AbbVie and Genmab’s recently approved bi-specific antibody in a second cancer indication positions them to talk to regulators about filings to challenge Roche’s Lunsumio.
The company halted studies of the treatment for a rare, advanced form of eye cancer after clinical results showed just one response in 47 patients.
The funds will support a trial of a tuberculosis vaccine developed by GSK that could potentially be the first new TB shot in a century.
Providing extra benefits and perks to parents in the workplace can be polarizing, but it doesn’t have to be.
After a series of milestone approvals in the first half of 2023, the FDA is slated to decide on four more firsts before the year’s end.
While early, the Phase I study results for 12 patients represent a promising return on Bayer’s investment in BlueRock, which it launched with Versant Ventures in 2016 and fully acquired three years later.
Overcoming an FDA rejection in January 2022, Pfizer and OPKO’s Ngenla will provide a long-acting, reduced-frequency treatment option for children with growth hormone deficiency.
The U.S. Equal Employment Opportunity Commission settled with Eli Lilly for $2.4 million after filing a suit in September over the company’s recruitment of younger workers.
Citing issues with a third-party contractor, the FDA rejected Regeneron’s regulatory application for a higher-dose regimen of Eylea, the company’s blockbuster eye injection treatment.
Despite a challenging economic climate and gloomy forecast, 2023 has still notched some mega-deals for biopharmas. BioSpace highlights the biggest deals in the industry this year.
The biopharma’s monoclonal antibody is the first to win approval for the two most common forms of generalized myasthenia gravis, a rare autoimmune and neuromuscular disorder.
Phase II results of MoonLake’s sonelokimab suggest superiority to the competition. Funds raised in the stock offering will support Phase III trials with an anticipated launch in 2027.
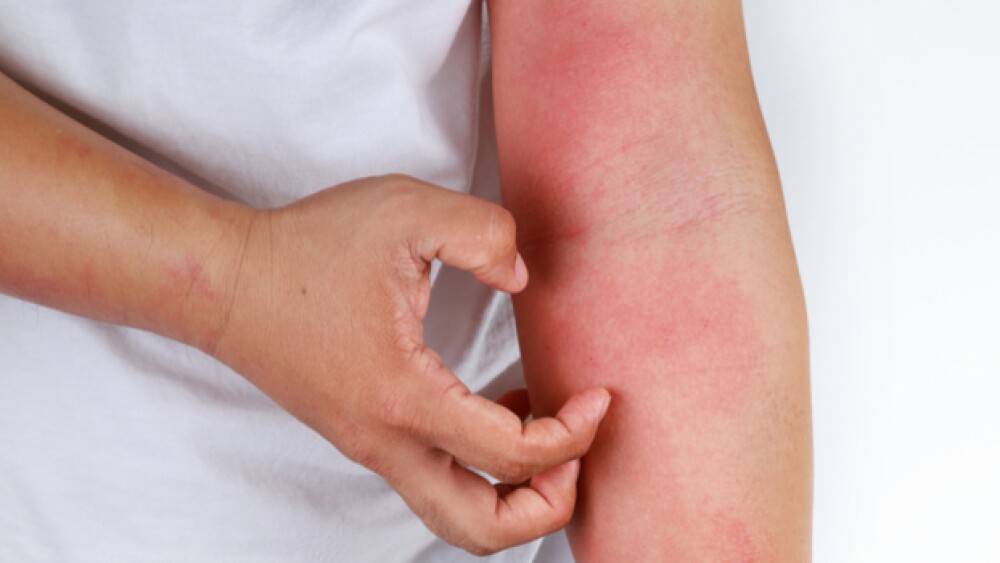
Though the topline data were sparse, Sanofi’s potentially first-in-class candidate amlitelimab improved Eczema Area and Severity Index scores in patients with moderate-to-severe atopic dermatitis.