All News
The U.S. regulator Monday approved the Beyfortus single-dose monoclonal antibody, developed jointly by the two companies, for the pediatric prevention of respiratory syncytial virus.
Part B of the study, cleared to proceed in Canada, remains on hold in the U.S. due to findings from non-clinical chronic toxicology studies.
The Swiss pharma is expanding its neuroscience pipeline with an upfront $500 million payment to DTx Pharma and additional payments of up to $500 million upon completion of certain milestones.
Following disappointing topline data, BridgeBio’s acoramidis has scored a late-stage win in transthyretin amyloid cardiomyopathy, leading to significant survival and quality of life benefits.
The Federal Trade Commission is asking for more information regarding Pfizer’s planned $43 billion acquisition of Seagen, according to the latter’s Securities and Exchange Commission filing on Friday.
Full data for Eli Lilly’s Phase III TRAILBLAZER-ALZ 2 study, presented Monday at the 2023 Alzheimer’s Association International Conference, confirm positive results announced in May.
This week: Cancer license deals from J&J and BeiGene, a potential $7B acquisition by Roche and confirmed $1.9B Lilly buy, EU fine for Illumina, and more legal challenges to the Inflation Reduction Act
Eli Lilly said Friday it plans to pay up to $1.925 billion to acquire Versanis and its lead asset, bimagrumab, a monoclonal antibody that aims to reduce fat mass without affecting muscle mass.
Bringing new drugs to the market costs billions of dollars. It could not be done without investments by both the NIH and biopharma companies.
The Swiss pharma is in talks to acquire Roivant Sciences’ RVT-3101, an anti-TL1A antibody that recently showed promising results in a Phase IIb ulcerative colitis trial, reports The Wall Street Journal.
A new report by the U.S. Government Accountability Office finds that despite the promise of regenerative medicine technologies they are held back by regulatory and manufacturing challenges.
In a Phase I trial, Caribou’s allogeneic CAR-T cell therapy candidate induced high rates of treatment response in patients with relapsed or refractory B cell non-Hodgkin lymphoma.
After beginning July with a splash in the Alzheimer’s space, the FDA is expected to make three decisions during the next two weeks; two for cancer and one for a viral skin disease.
If you’re on the hunt for a new role, here are five things to look out for that may be indicative of a toxic work environment.
For over three years, no interest has accrued on federal student loans and no payments were required. Soon, mandatory payments will resume, leaving workers with less money in the bank.
Recent data from the Phase III study of donanemab emphasize a correlation between amyloid and tau. Experts say a greater understanding of this link could further Alzheimer’s drug development.
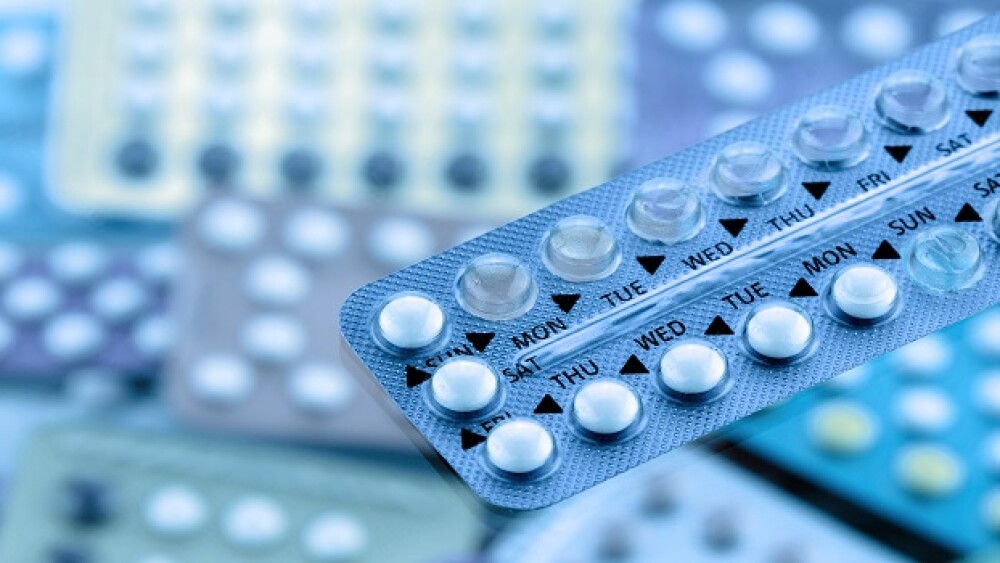
The regulator on Thursday approved Opill, the first oral contraceptive available in the U.S. without a prescription. Perrigo shares rose 6% in response to the news.
Data from the OCARINA II trial shows that a 10-minute subcutaneous injection of Ocrevus achieves similar pharmacokinetics as the typical hours-long intravenous infusion in multiple sclerosis patients.
Following its initial lawsuit last month, Merck has now requested that a federal judge rule on its Inflation Reduction Act case against the Biden administration without a trial.
As the market recovers from the effects of the COVID-19 pandemic, employers are beginning to regain some of the power they lost in recruiting, forcing candidates to adjust their asks.