Vaccines
Although COVID-19 appears to be on the run in the U.S., there is still a threat of resurgence. Here’s a look at some of the most recent COVID-19 stories and research.
On Wednesday, the FDA approved Merck’s pneumococcal 15-valent conjugate vaccine for children 6 weeks through 17 years of age.
The week began with positive updates in the vaccine development space against various infectious diseases from Emergent, Merck, Affinivax and Ocugen.
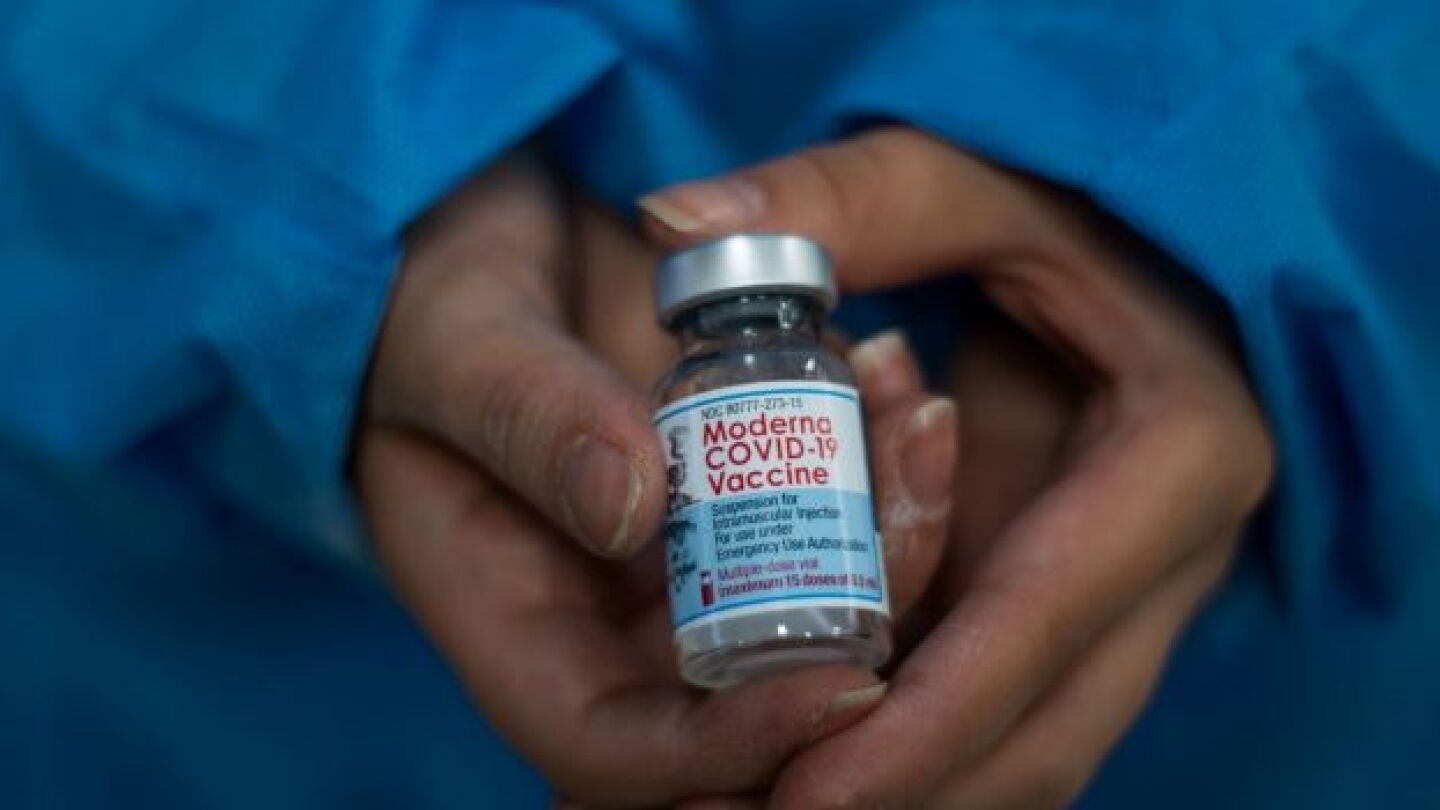
Thursday, a committee of advisors for the CDC voted unanimously for children and teens, ages six to 17 to receive Moderna’s COVID-19 vaccine.
Pfizer inked an Equity Subscription Agreement with France-based Valneva. They also updated their Collaboration and License deal for a Lyme disease vaccine that was announced in April.
Friday morning, the FDA granted Emergency Use Authorization to both Moderna’s and Pfizer-BioNTech’s COVID-19 vaccines for use in children ages 6 months to 4 years old.
The World Trade Organization approved vaccine patent waivers to increase the availability of COVID-19 vaccines to lower-income countries.
Moderna’s study, dubbed BabyCove, is expected to begin recruiting in September and will include up to 700 babies three to six months of age.
An FDA advisory committee voted Wednesday to recommend both the Pfizer-BioNTech and Moderna COVID-19 vaccines for children as young as six months old.
Clover dosed the first participants in a Phase III study of its COVID-19 booster shot, while Global Access Diagnostics, Orbit Discovery, Proximie and more provide business and pipeline updates.
PRESS RELEASES