Infectious disease
Current COVID-19 booster shots have a problem: they last only about four months and appear to have limited efficacy in a vaccinated population. Clearly, a more durable approach is needed.
Despite posting its first profitable quarter as a commercial stage company, shares of Novavax plunged 20% in Tuesday trading due to the slower-than-expected rollout of its COVID-19 vaccine.
Moderna is still fending off patent challenges over the lipid nanoparticle delivery system used in its COVID-19 vaccine.
Life Science industries are seeing massive financial fluctuations in how they’re handling the COVID-19 pandemic, but there is some optimism of a recovery by biopharma executives.
Despite having infinitely better global communications, exact figures are hard to come by for COVID-19 fatalities. Here’s a look at that story and more COVID-19 news.
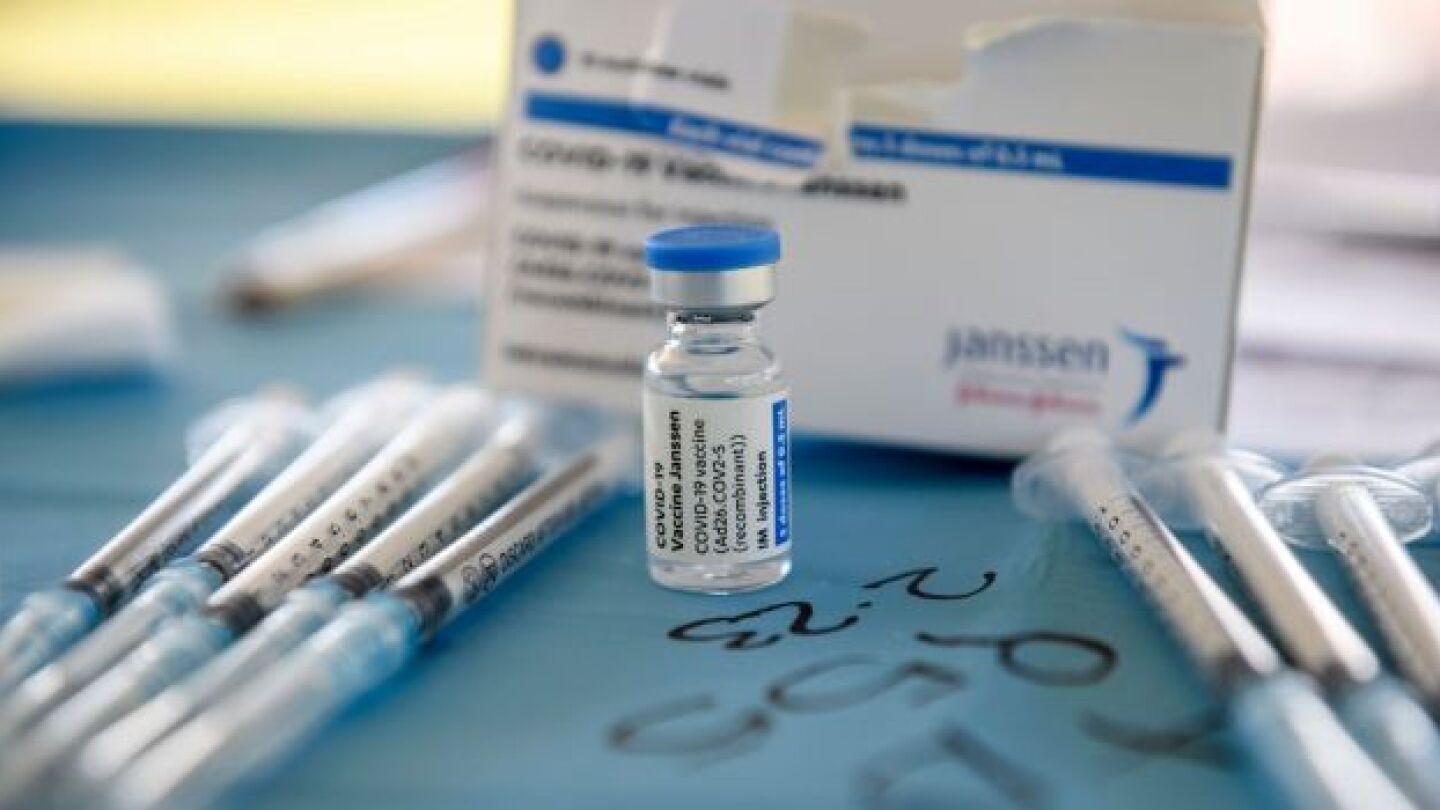
Janssen’s COVID-19 vaccine is now limited to certain individuals ages 18 years and up after the FDA downgraded its emergency use authorization (EUA).
The FDA’s reaction came after Pfizer CEO Albert Bourla said that physicians may give a second five-day course of Paxlovid if patients see a spike in their SARS-CoV-2 viral load.
New studies suggest that the virus may aggravate childhood asthma after infection. For that and more COVID-19 news, continue reading.
Moderna held its 2022 Q1 earnings call this morning announcing $6.1 billion in revenue and robust plans to roll out COVID-19 boosters this fall.
Three long-time FDA officials, Dr. Peter Marks, Dr. Janet Woodcock and Dr. Robert Califf wrote an op-ed in JAMA describing the reality that COVID-19 represents “the new normal.”
PRESS RELEASES