Sage Therapeutics
Despite missing out on an FDA approval for major depressive disorder, Zurzuvae appears to be a strong asset for Sage Therapeutics, with high growth potential in 2024.
With recent scientific advances, milestone approvals and increased dealmaking, the future of treatment for neurological diseases looks brighter—but continued investment, collaboration and patient-focused efforts are key.
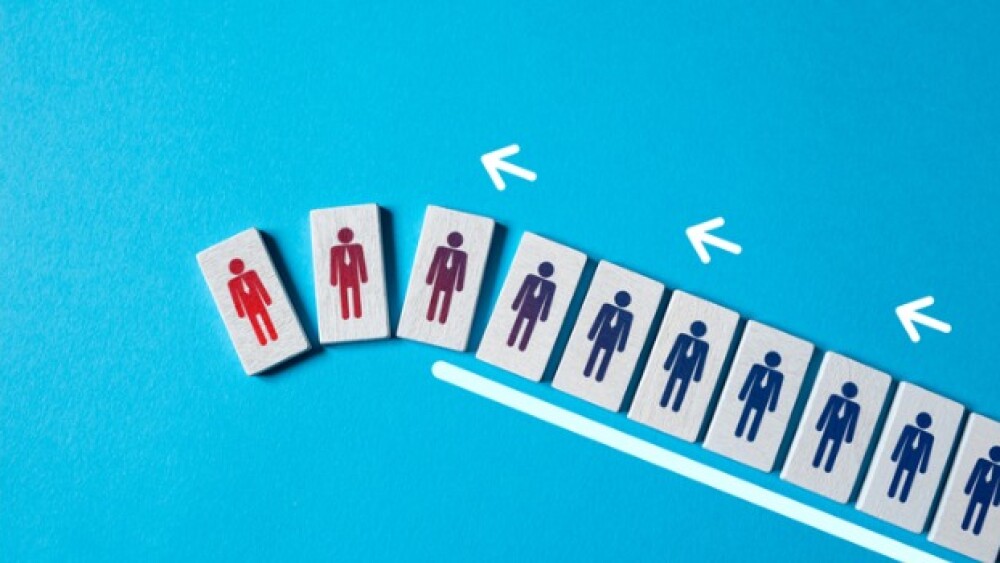
$27.8B Amgen-Horizon deal gets FTC clearance with restrictions; the White House names first 10 drugs subject to Medicare price negotiations; Sage Therapeutics axes 40% of staff.
Following the FDA’s recent rejection of zuranolone in major depressive disorder, Sage Therapeutics has launched a strategic reorganization initiative including a 40% reduction in headcount.
As the FDA’s recent rejection of Biogen and Sage’s zuranolone for major depressive disorder highlights, biopharma companies will need to tackle emerging challenges to bring more of these drugs to patients.
With a potential $509 billion up for grabs by 2028, companies including Biogen, Sage, Karuna Therapeutics and Cerevel Therapeutics are vying to bring their drugs across the regulatory finish line.
The FDA approved Sage and Biogen’s zuranolone Friday as the first oral medication for postpartum depression but declined to approve the application in major depressive disorder.
A transformational moment in the treatment of depression, GSK takes first shot in a vaccine patent war with Pfizer, a Louisiana woman sues Novo Nordisk and Lilly, and companies face a steep COVID-19 cliff.
Partners Biogen and Sage Therapeutics had sought approval for the therapy in both postpartum depression and major depressive disorder, but the FDA rejected the application for the latter.
JOBS
IN THE PRESS