Abbott Laboratories
NEWS
The Basel area is home to over 800 life sciences companies, including Novartis and Roche, according to nonprofit Basel Area Business & Innovation. The nonprofit’s CEO and a BeOne Medicines executive discuss the location’s evolution, advantages and future.
Between top-notch academic institutions, solid venture capital funding, expanding lab space and governmental support, Chicago is emerging as a true hotbed for biotech growth.
The FDA is keeping busy as summer winds down, with approvals, Orphan Drug Designations and other actions. Here’s what the agency has been up to this week.
The WHO reports it’s too early to tell if the monkeypox outbreak could become a pandemic, but believe there is a window of opportunity to control the cases.
The crisis caused by the shutdown of one plant is a stark reminder of critical supply chain issues in other sectors, including the pharmaceutical industry.
More pharmaceutical companies are joining to put economic pressure on Russia by suspending some or all operations within that country following the invasion of Ukraine.
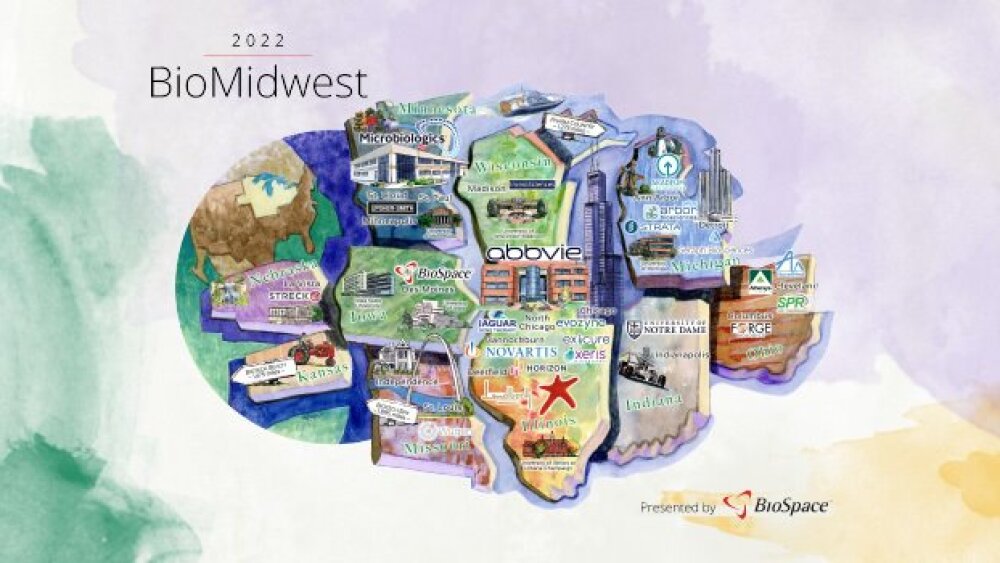
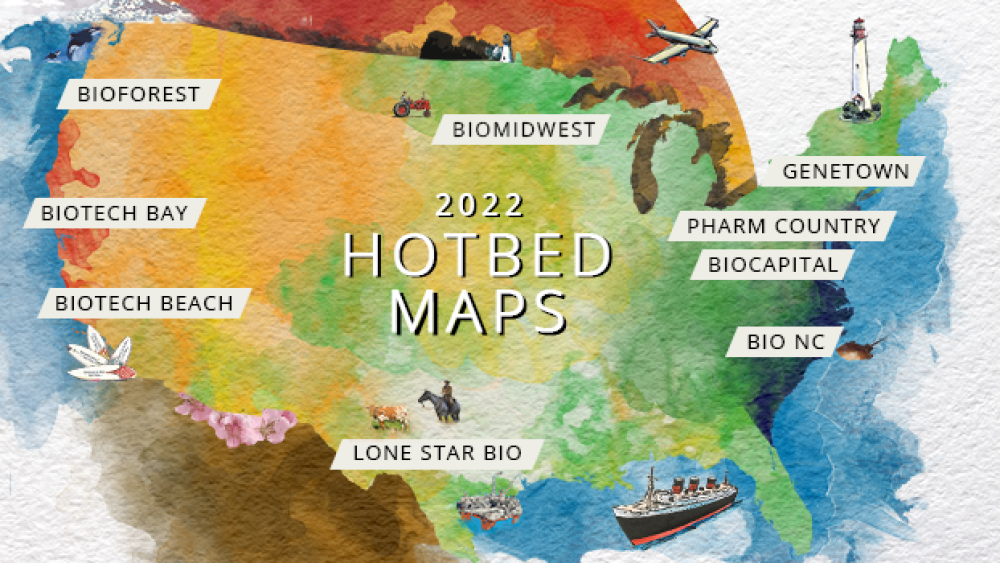
As BioSpace proudly introduces our 2022 Hotbed Maps, let’s explore the industry’s most thriving territories, research leading employers and search for relevant jobs on BioSpace.
On June 26, Intellia announced the first-ever clinical data supporting the safety and efficacy of in vivo CRISPR genome editing in human patients.
For the past year, Abbott Laboratories and Quest Diagnostics saw their revenue driven by COVID-19 diagnostics, but that seems to be changing as more and more vaccinated individuals are seeking medical advice for non-viral related issues.
JOBS
IN THE PRESS