Sarepta Therapeutics, Inc.
NEWS
Wrapping up the month of August, there are technically four PDUFA dates on the calendar, although one was approved two months early and another may be delayed as the U.S. Food and Drug Administration reevaluates its policies regarding opioid pain medications. Here’s a look.
It was reported that a boy in Sarepta Therapeutics clinical trial for its micro-dystrophin gene therapy for Duchenne muscular dystrophy had a serious adverse event. However, the company indicates the adverse event report was submitted erroneously.
The company, best known for Exondys 51 (eteplirsen) for Duchenne muscular dystrophy (DMD), reported a net loss of $276.4 million for the quarter compared to a loss of $109.3 million in the same period in 2018. For the six-month period ending June 30, Sarepta reported a net loss of $353 million.
It was a typically busy week for clinical trials, with announcements in a wide variety of indications. Here’s a look.
As the largest center for biotech startups in the U.S., the Boston/Cambridge, Massachusetts area often acts as a marker for the entire industry. Here’s a look at the Massachusetts M&A deals so far.
All three studies found significant slowing of respiratory decline in the patients receiving Exondys 51 and it was consistent across all stages of the disease studied.
Sarepta struck a deal with the Research Institute at Nationwide Children’s Hospital for the gene therapy candidate, calpain 3.
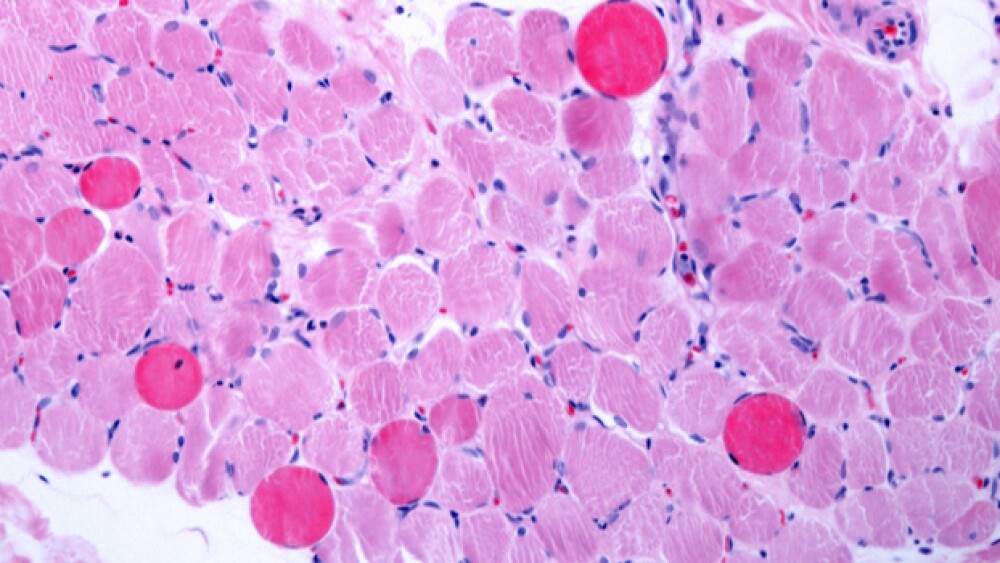
Cambridge, Mass.-based Sarepta Therapeutics announced data from its interim analysis of muscle biopsy endpoints of its therapy casimersen for Duchenne muscular dystrophy (DMD). The interim data was strong enough to support a probable New Drug Application (NDA) submission to the U.S. Food and Drug Administration (FDA) by mid-year.
As part of the deal, Sarepta picks up five gene therapy candidates to treat distinct types of LGMD. The original deal was inked in May 2018, when the two companies partnered to develop five gene therapy compounds.
JOBS
IN THE PRESS