Sanofi (France)
NEWS
Sanofi announced plans to increase its vaccines research and production capabilities. As part of the plan it will invest $679.4 million (€610 million) to create a new production site and a research center, both in France.
It was a fairly busy week with clinical trial updates and announcements. Here’s a look.
After Disappointing Study Results, Denali and Sanofi Shift Resources to a Different Alzheimer’s Drug
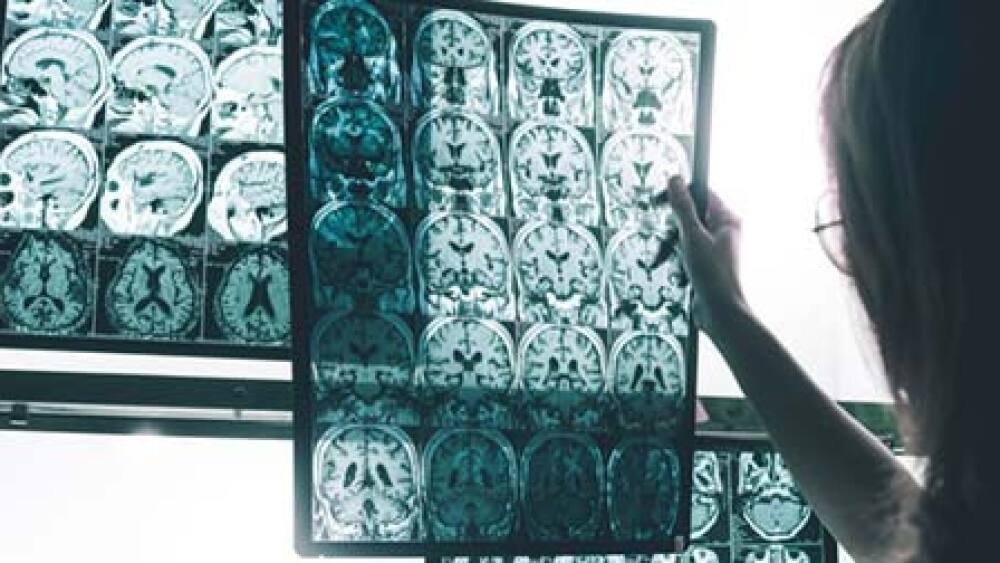
Denali Therapeutics and its development partner Sanofi announced data from its Phase Ib trial of DNL747 in Alzheimer’s disease and amyotrophic lateral sclerosis (ALS).
Biopharma and life sciences companies from across the globe provide updates on their pipelines and businesses.
After four years as head of Sanofi’s vaccines division, David Loew is departing the pharma giant to take on a new role – chief executive officer of Paris-based Ipsen, effective July 1.
To date, Dupixent is the only biologic approved for this age group.
Sanofi is selling off its nearly $13 billion in investments in longtime development partner Regeneron as the France-based company shifts its strategy to focus on indications such as oncology, neurology, rare diseases and hematology.
Heading into the first week after Memorial Day, which in the U.S. marks the typical beginning of summer, the U.S. Food and Drug Administration has a number of drug approvals on the calendar. Here’s a look.
Clinical trial updates not related to COVID-19 are on the upswing, partly because some companies are announcing trial information ahead of the upcoming American Society of Clinical Oncology virtual meeting being held at the end of the month. Here’s a look.
JOBS
IN THE PRESS