Sanofi (France)
NEWS
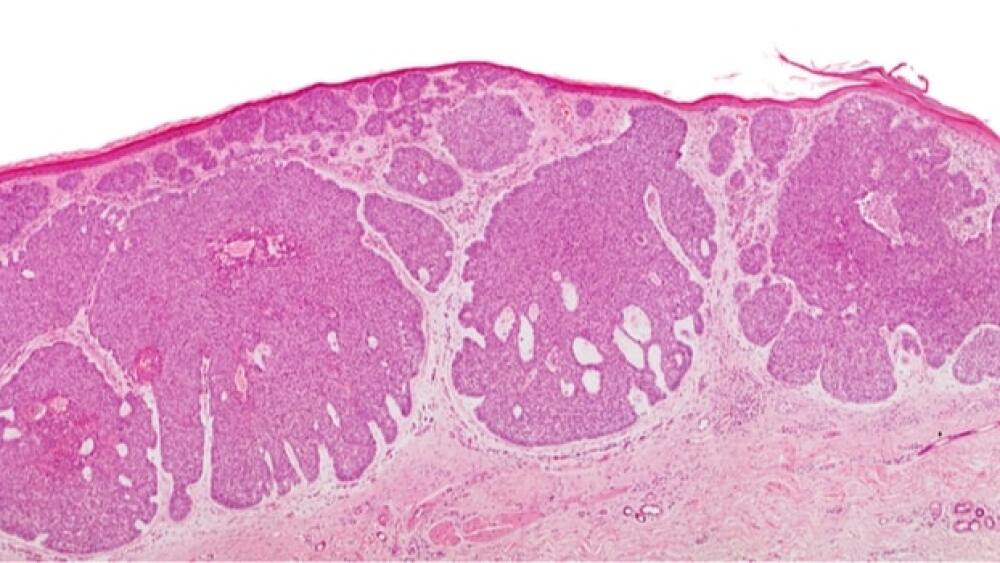
Regeneron and Sanofi broke new ground in treating advanced basal cell carcinoma with their anti-PD-1 inhibitor, Libtayo.
The CEOs of AstraZeneca, BioNTech, GlaxoSmithKline, Johnson & Johnson, Moderna, Novavax, Pfizer, Merck and Sanofi all signed the pledge promising to ensure that any vaccine put forth by one or more of the companies meets the rigorous standards for approval.
Results from a Phase III open-label extension trial showed that the safety and efficacy profile observed in previous Dupixent trials were maintained for up to three years in adults and adolescents with moderate-to-severe asthma.
It was a busy week for both COVID-19-related clinical trial news and the rest of the industry. Here’s a look.
Another Kevzara study in hospitalized COVID-19 patients failed to meet primary and secondary endpoints.
In a regulatory filing with the U.S. Securities and Exchange Commission, Translate Bio indicated that the COVID-19 vaccine it is developing with Sanofi induced an immune response in non-human studies.
Under terms of the deal, Sanofi will acquire all of the outstanding shares of Principia for $100 per share in cash, which represents an aggregate equity value of approximately $3.68 billion.
Three months after joining forces to develop an adjuvanted vaccine for COVID-19, Sanofi and GlaxoSmithKline reached an agreement with the U.K. government to provide up to 60 million doses of the preventative medication.
Sanofi is lining up to spin off its active pharmaceutical ingredients division that will include a listing as a public company, Reuters reported, citing unnamed sources.
JOBS
IN THE PRESS