COVID-19
Please check out the biopharma industry’s COVID-19 stories that are trending for April 27, 2021.
A new study published online has provided some hope for the citizens in India, with researchers suggesting that the Covaxin vaccine, developed by Bharat Biotech and Pennsylvania-based Ocugen, could neutralize the SARS-CoV-2 variant leading the second wave in the country.
Sanofi will use its manufacturing capabilities to support the development of 200 million doses of the Moderna COVID-19 vaccine beginning in September.
Leaked documents from provincial and municipal governments in China reveal a slew of previously unreported severe adverse events related to COVID-19 vaccines made and administered in China.
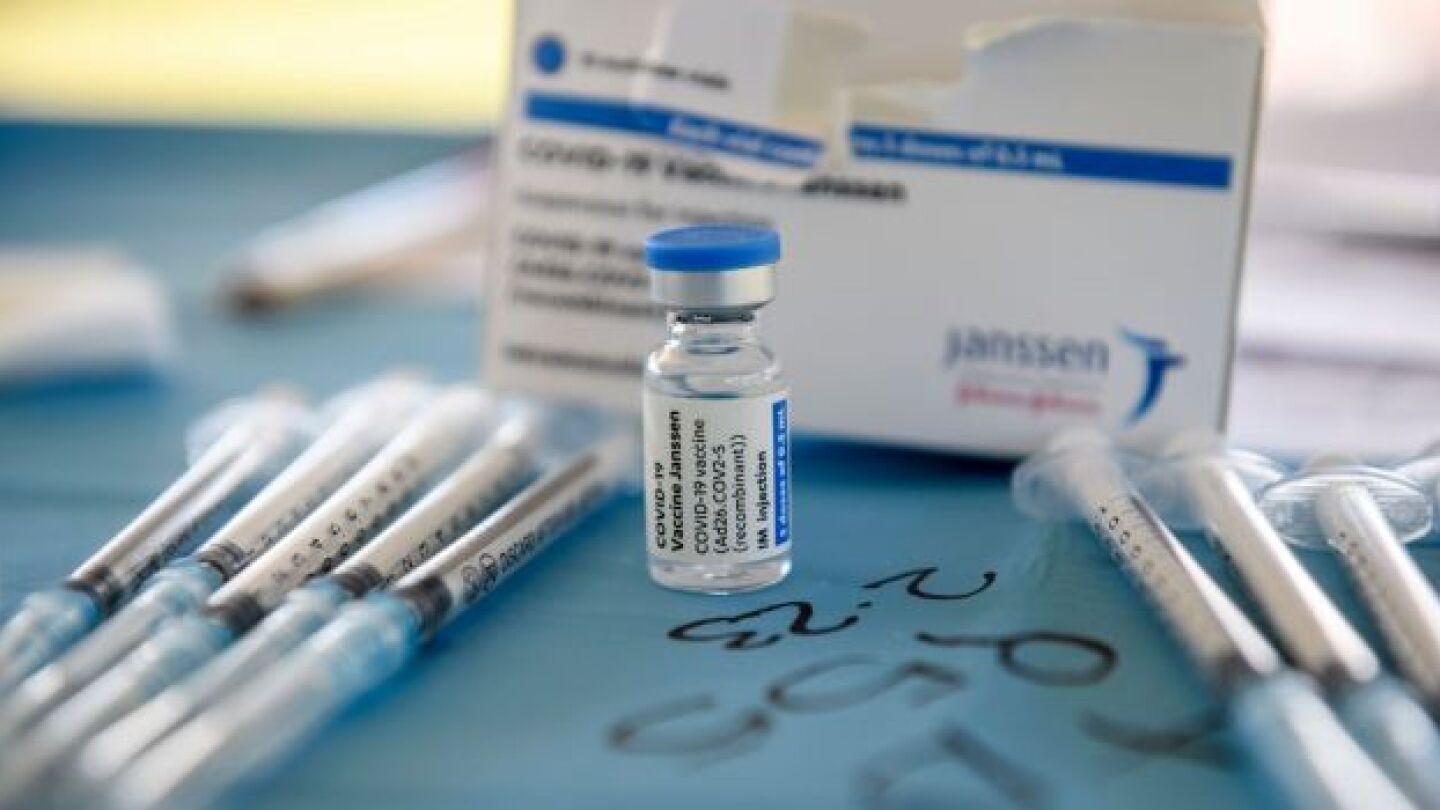
On Friday April 23, the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) lifted the restrictions on the Johnson & Johnson COVID-19 vaccine.
With the U.S. Food and Drug Administration’s vaccines advisory committee meeting being held today to discuss the Johnson & Johnson vaccine and blood clotting, there’s even more interest in what’s going on in the pandemic than usual. Here’s a look at some of the top stories.
It was a busy week for non-COVID-19-related clinical trial news, while fairly quiet on the COVID-19 arena. Here’s a look.
Molecular Partners announced yesterday it has filed for a $100 million initial public offering with the Securities and Exchange Commission in the U.S., funding which will go toward supporting the company’s work in the development of protein-based treatments for COVID-19 and various cancers.
A new study suggests that many patients with long-term COVID-19 symptoms still experience health issues six months following infection, are survivors with “long haul” disease have a greater risk of dying and use a more significant number of medications than patients who have fully recovered from the virus.
Ocugen’s plan to bring a COVID-19 vaccine developed by India-based Bharat Biotech later this year remains on track following an interim analysis of Phase III data that shows the vaccine demonstrated a 78% efficacy against mild to moderate infection and 100% efficacy against severe COVID-19.
PRESS RELEASES