Clinical research
The FDA recently approved the first cellular therapy for Type 1 diabetes and others may not be far behind. But experts say challenges still exist to the widespread application of these treatments.
The company’s ruxolitinib cream Opzelura led to higher rates of treatment success in children with atopic dermatitis than with a control cream.
Severe respiratory problems killed seven patients and affected five others, though the company said that most of these were likely unrelated to the drug.
After paying $300 million upfront to BeiGene for option rights to ociperlimab in December 2021, Novartis has dropped the agreement and given the rights back to the Chinese biotech.
Early-stage data shows that Viridian’s thyroid eye disease candidate induces clinically meaningful improvements in eye protrusion after six weeks of treatment.
After its Biologics License Application was rejected by the FDA, BrainStorm’s Phase III data suggest its amyotrophic lateral sclerosis candidate significantly lowers neurofilament light chain levels.
Johnson & Johnson has licensed Nanobiotix’s lead radioenhancer candidate, designed to increase the efficacy of radiation treatment in cancers, capitalizing on the latter’s rocky financial situation.
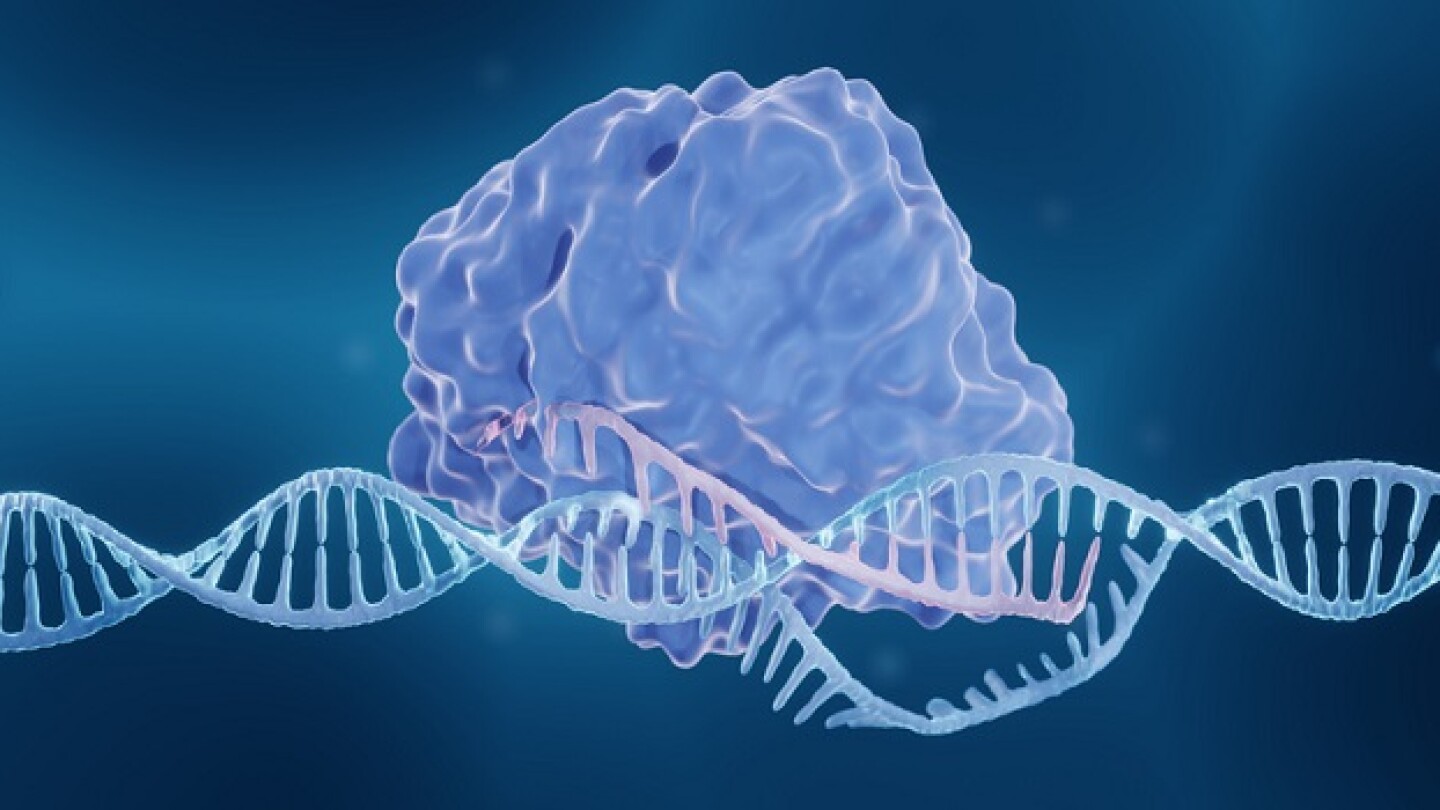
Gene editing technologies are advancing rapidly in the clinic, with the potential first approval later this year, but challenges remain.
Phase III trial data published Thursday show significant survival benefit as a first-line treatment in advanced non-small cell lung cancer patients not fit for standard platinum-based doublet chemotherapy.
The European Medicines Agency recently flagged a safety signal related to GLP-1 receptor agonists and sent a list of questions to manufacturers including Novo Nordisk, Eli Lilly, Sanofi and AstraZeneca.
PRESS RELEASES