All News
On 25 January, Lonza Bioscience Informatics and NNIT will host a free 60-minute webinar to discuss the key requirements of next generation Manufacturing Execution Systems (MES) and how small- and medium-sized companies can overcome critical implementation challenges for accelerated pharma digitalization.
The SPAC announced it is merging with medical technology company ProKidney LP, in a deal that values the combined equity of the companies at $2.64 billion.
Moderna is poised to commercialize its COVID-19 and influenza combination vaccine by the fall of 2023, with some potential for the same drug to also be viable against the RSV.
The agency recommended another clinical trial be conducted to confirm the data from the study on the 3.2-mg dose of the intranasal carbetocin for Prader-Willi syndrome.
Amagma Therapeutics announced a licensing agreement with Innovent Biologics for up to three enzyme specific inhibitors derived from Amagma’s proprietary SEIZMIC Platform.
Based on the success of a joint immuno-oncology antibody collaboration program targeting CD96, GlaxoSmithKline and 23andMe have extended their 2018 collaboration for an additional year.
The company will continue to analyze data regarding the secondary endpoints.
The extensions are due to the FDA’s requiring more time to review additional clinical data it had requested from bluebird.
The decision to carry on came after dosing for the Phase III GENERATION HD1 trial was halted following an overall risk/benefit assessment.
Evotec entered into a drug discovery collaboration for metabolic diseases with Eli Lilly, focusing on kidney disease and diabetes.
Studies are ongoing in Israel on whether a fourth dose of an mRNA vaccine offers additional protection. Although the data is still early, it suggests that it might not.
The problem Kinaset is trying to solve is that over time, patients receiving asthma therapies – typically inhaled corticosteroids plus bronchodilators – become steroid-refractory.
While many of these scams targeted the public at large, scammers also turned their sights to those sources that would naturally be on the spot as the result of demand.
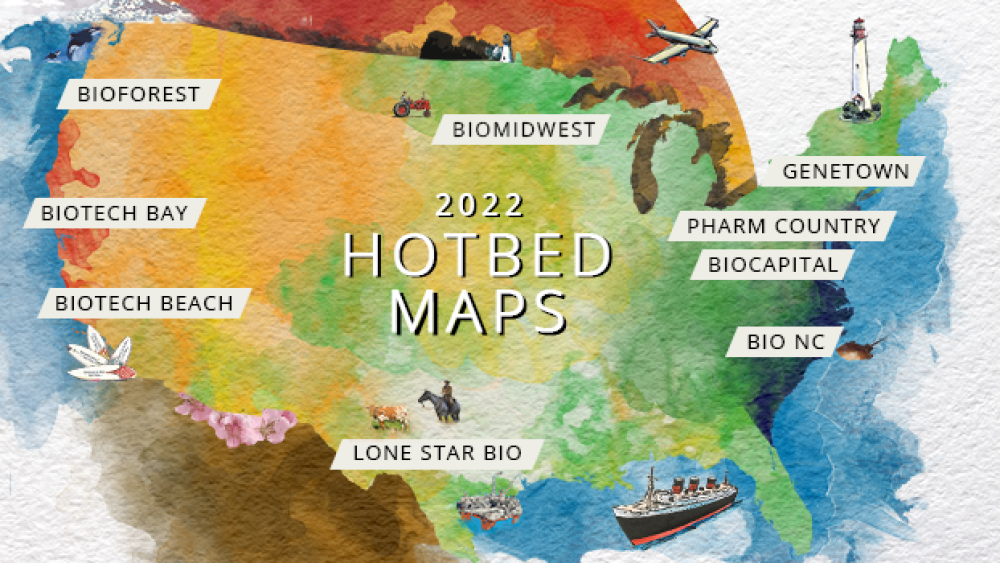
As BioSpace proudly introduces our 2022 Hotbed Maps, let’s explore the industry’s most thriving territories, research leading employers and search for relevant jobs on BioSpace.
The new building is expected to be ready for occupation in the fourth quarter of 2022 and will feature purpose-built laboratory infrastructure, full-service onsite amenities and multi-functional outdoor spaces.
Expanded approval for Rinvoq was based on three Phase III studies that included more than 2,500 patients. The studies met all primary and secondary endpoints.
The Danish pharma giant is partnering with EraCal Therapeutics, to develop new obesity-related drug targets.
BioSpace is marking Martin Luther King, Jr. Day by sharing some of our recent coverage about what the life sciences industry is doing to address racial inequality.
The approval of Cibinqo was based on data from five clinical trials of more than 1,600 people. Safety and efficacy were studied in three Phase III trials.
Martin Shkreli and his former company, Vyera Pharmaceuticals, earned a 5,000% increase on the toxoplasmosis drug, Daraprim.