bluebird bio
NEWS
Although the beginning of March was fairly slow, the end of the month shows a busy schedule for PDUFA dates for the U.S. FDA. Read on to see what’s on the calendar for this week.
Another bluebird bio executive is jumping ship. Chief Medical Officer David Davidson is leaving the company on April 16.
It was another busy week for clinical trial announcements. Here’s a look including trials for COVID-19, migraine, Parkinson’s disease, Alzheimer’s, HIV and more.
The European Medicines Agency has launched a safety review of bluebird bio’s thalassaemia drug Zynteglo, a conditionally licensed gene therapy in Europe.
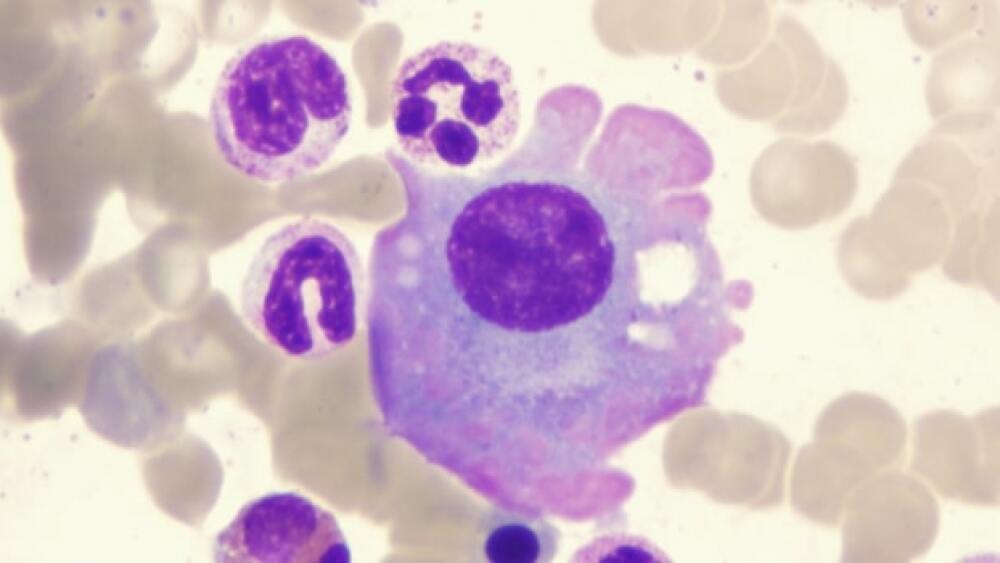
Preliminary findings of LentiGlobin suggested that the BB305 LVV vector was present in the AML blast cells, but there was not sufficient information to determine causality.
Bristol Myers Squibb and bluebird bio said data from the Phase II KarMMa study evaluating the safety and efficacy of ide-cel (idecabtagene vicleucel) met the primary endpoint of overall survival and the key secondary endpoint of complete response rate.
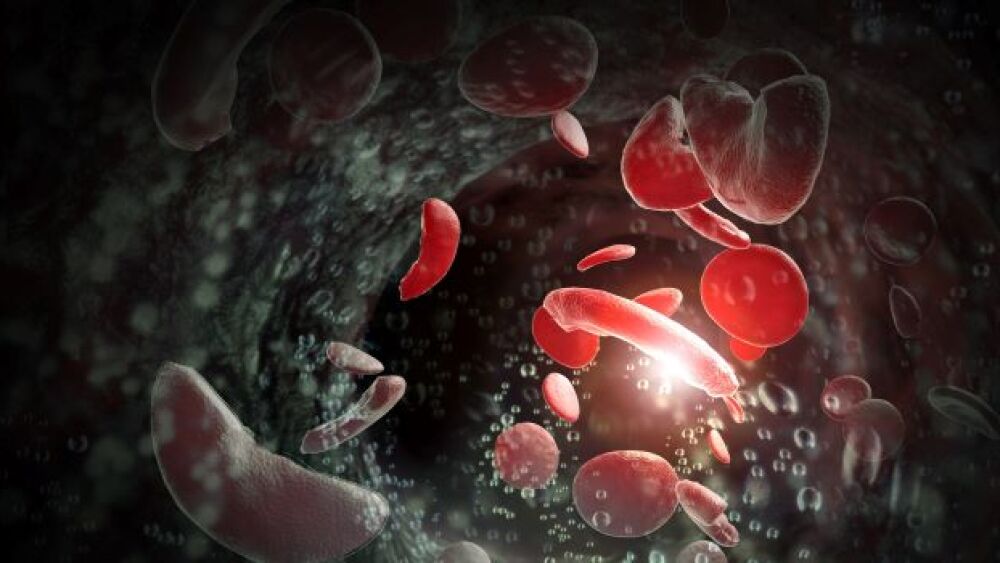
The hold comes just after patient dosing began in HGB-210, the company’s Phase III single-arm open-label LentiGlobin trial for SCD patients between the ages of 2 and 50.
Bluebird bio announced that it has placed its Phase I/II and Phase III trial of LentiGlobin gene therapy for sickle cell disease (SCD) on temporary suspension.
Bluebird bio announced plans to split its genetic disease and oncology businesses. Bluebird bio will stay focused on severe genetic disease and spin out its oncology business into a new company.
JOBS