BioNTech
NEWS
They caution that as more data is accumulated and analyzed, the efficacy percentage may shift. Nonetheless, it’s extremely promising and there were no serious safety signals.
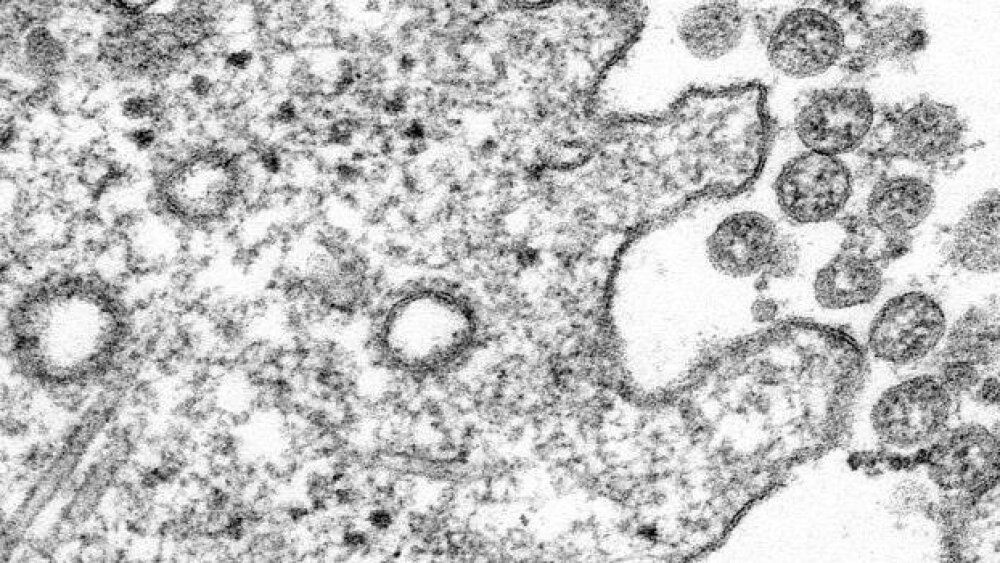
Several biopharmaceutical companies have made headway as of late in the race to develop a vaccine for COVID-19, which is responsible for the current global pandemic.
Allele Biotechnology and Pharmaceuticals filed lawsuits claiming the companies infringed on Allele’s patented mNeonGreen technology in the development of their COVID-19 treatments.
A few days ago, CNBC reported that five patients, three in Moderna’s and two in Pfizer’s Phase III trials, had experienced more severe, although transient side effects.
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for October 6, 2020.
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for September 18, 2020.
The manufacturing site will expand BioNTech’s COVID-19 vaccine production capacity by up to 750 million doses per year, or over 60 million doses per month, once fully operational, the company said.
Only days after saying it expects to have key data from its Phase III clinical trial of its COVID-19 vaccine in October, Germany’s BioNTech received a grant of up to €375 million from the German Federal Ministry of Education and Research.
In a weekend interview on CBS’ “Face the Nation,” Albert Bourla, chief executive officer of Pfizer indicated they may have key data from its Phase III trial of the vaccine to the U.S. Food and Drug Administration by the end of October.
JOBS
IN THE PRESS