Food and Drug Administration (FDA)
NEWS
BeiGene’s candidate drug tislelizumab for cancer succeeded in meeting the primary endpoint of overall survival in a global Phase III trial. Learn more about it here.
The U.S. Congress is calling for the coronavirus management plan to deliberate on the timeline for the approval and release of vaccines for children under 5 years old.
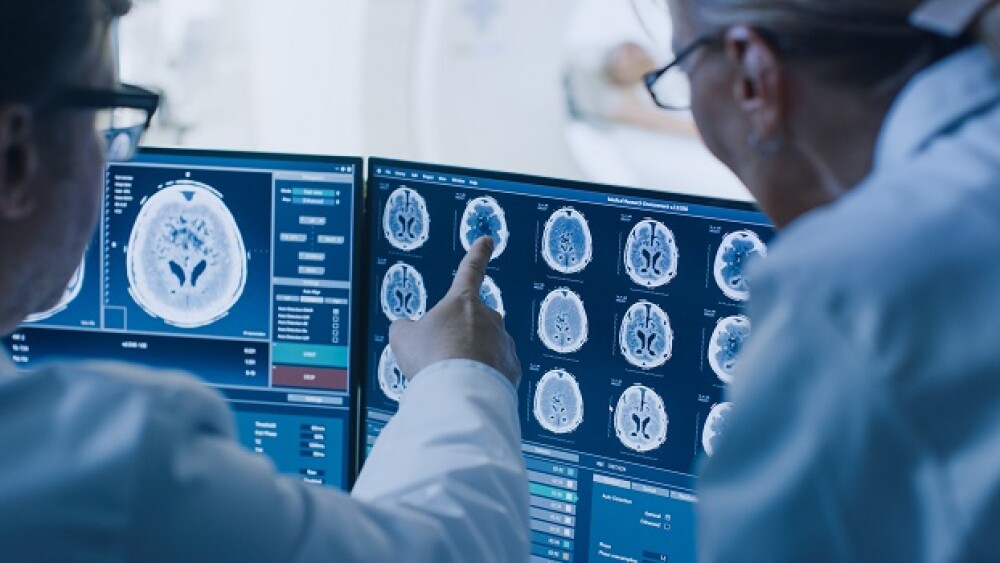
New research suggests that treatment with anti-amyloid-beta protofibril antibody lecanemab is estimated to slow the rate of disease progression in patients with Alzheimer’s Disease.
Editas Medicine snagged Rare Pediatric Disease designation for its experimental beta thalassemia gene therapy, while VBL received Fast Track designation for an ofra-vec combination in ovarian cancer.
How many boosters are enough to protect against COVID-19 infection? This question has been at the forefront of the minds of everyone as news crops up about yearly boosters.
Gamida Cell reported that the FDA had lifted its clinical hold on its cryopreserved formulation of GDA-201. It expects to launch a Phase I/II drug trial for those indications this year.
Pfizer and its partner, Valneva, have announced positive Phase II data from its trial of vaccine candidate VLA15 in a pediatric population. VLA15 is intended to prevent Lyme Disease (LD).
The FDA has now approved the first treatment for COVID-19 in young children, expanding its approval for Gilead’s Veklury to children who are at least 28 days old and weigh at least three kilograms.
Shares of Axsome Therapeutics have fallen more than 20% in trading this morning after the company announced the FDA is unlikely to approve its acute migraine treatment AXS-07 due to unresolved quality control issues.
JOBS
IN THE PRESS