Food and Drug Administration (FDA)
NEWS
SonoCloud ultrasound device for glioblastoma patients will be tested for first time at US clinical sites
This announcement contains inside information for the purposes of Article 7 of the Market Abuse Regulation (EU) No 596/2014.
Da Volterra, a clinical-stage biopharmaceutical company developing new therapeutics to protect the intestinal microbiota, announced the completion of patient recruitment for its Phase 2 trial ‘SHIELD’ evaluating DAV132 in patients receiving antibiotics.
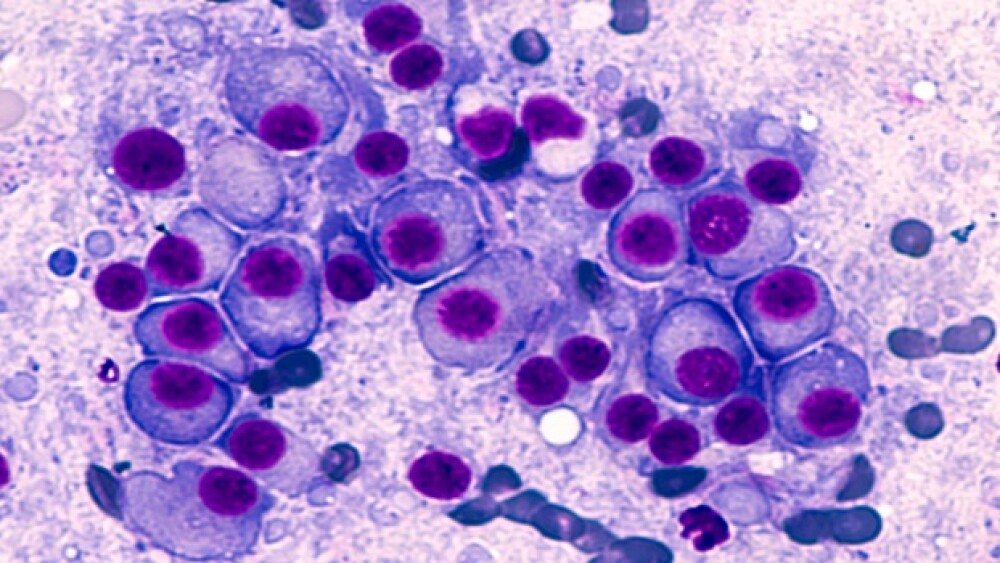
Shares of Karyopharm Therapeutics have skyrocketed more than 36% after the U.S. Food and Drug Administration approved the Newton, Mass.-based company’s treatment for multiple myeloma.
The latest clinical hold was placed after a patient experienced serious adverse events that included neurotoxicity and cytomegalovirus infection, as well as severe respiratory distress.
Genentech, a Roche company, announced that its Phase III MINISTONE-2 clinical trial hit its primary endpoint, showing that its Xofluza (baloxavir marboxil) was comparable to its own Tamiflu (oseltamivir). It was also well-tolerated in children with the flu.
Only days after it was announced that Scott Gottlieb, former commissioner of the U.S. Food and Drug Administration (FDA), was joining Pfizer’s board of directors, presidential hopeful Senator Elizabeth Warren (D-Mass) called for him to resign from the position.
Immigration has been a central concern of President Donald Trump. Since taking office, his administration has put forth a number of policies to restrict the number of immigrants allowed each year, as well as from what nations those immigrants can come.
Based on positive data from its FINCH program, Gilead aims to seek regulatory approval for its Jak inhibitor.
JOBS
IN THE PRESS