Food and Drug Administration (FDA)
NEWS
A U.S. FDA committee gave the nod to continue using Genentech’s checkpoint inhibitor Tecentriq in combination with chemotherapy to treat adults with mTNBC.
Enzyvant has resubmitted its Biologics Licensing Application to the U.S. FDA for its tissue-based regenerative pediatric congenital athymia therapy RVT-802.
There is hope for uniQure’s HOPE-B trial after all. Shares of uniQure NV are climbing this morning after the company announced the clinical hold on its hemophilia B gene therapy has been lifted by the U.S. Food and Drug Administration.
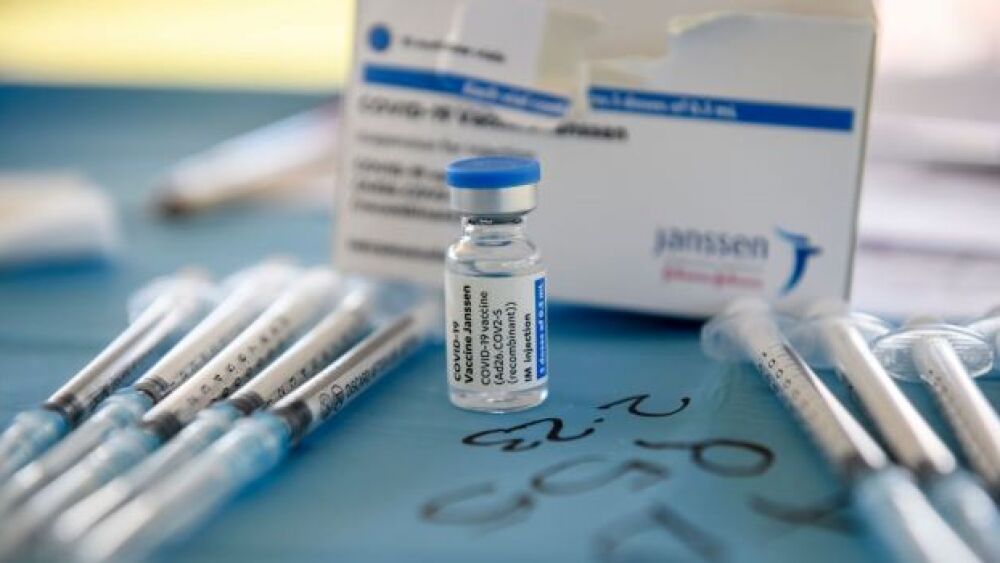
On Friday April 23, the U.S. Food and Drug Administration (FDA) and the Centers for Disease Control and Prevention (CDC) lifted the restrictions on the Johnson & Johnson COVID-19 vaccine.
The approval was specifically for patients with recurrent or advanced endometrial cancer who had progressed on or after previous treatment with platinum-based chemotherapy and whose cancers have a dMMR genetic anomaly.
The long and strange journey of Biogen’s Alzheimer’s drug aducanumab is coming to an end. Whether it will be a complete ending—with the U.S. FDA rejecting it—or a new chapter, with an approval, remains to be seen.
The U.S. FDA has requested that Emergent BioSolutions temporarily pause production of ingredients for the Johnson & Johnson COVID-19 vaccine at their facility in Baltimore.
This is an update on what is known about the clots and what the likely course of action is going to be.
Amgen announced the U.S. FDA awarded Breakthrough Therapy Designation to the bemarituzumab as a first-line treatment for certain types of gastric cancer.
JOBS
IN THE PRESS