Pfizer
NEWS
Pfizer’s oral antiviral treatment for COVID-19 is making its way through regulatory hurdles. The company is working with global manufacturers to ensure a steady supply of the medication.
Respiratory Syncytial Virus has been around for a very long period now. But there was no vaccine yet. But, here’s the first vaccine for RSV to shape the future.
This year’s top 30 large and small companies operate in some of the Life Sciences’ hottest spaces – precision medicine, vaccines, gene editing, genomics and oncology.
Between the numerous COVID-19 vaccines and several new antiviral drugs, it would appear that the tools to end the COVID-19 pandemic are within reach. Here’s a look at some of the top stories.
The total revenue for the quarter was $9.74 billion, a leap of 47%. The COVID-19 vaccine, Vaxzevria, only contributed 1% to the company’s profit.
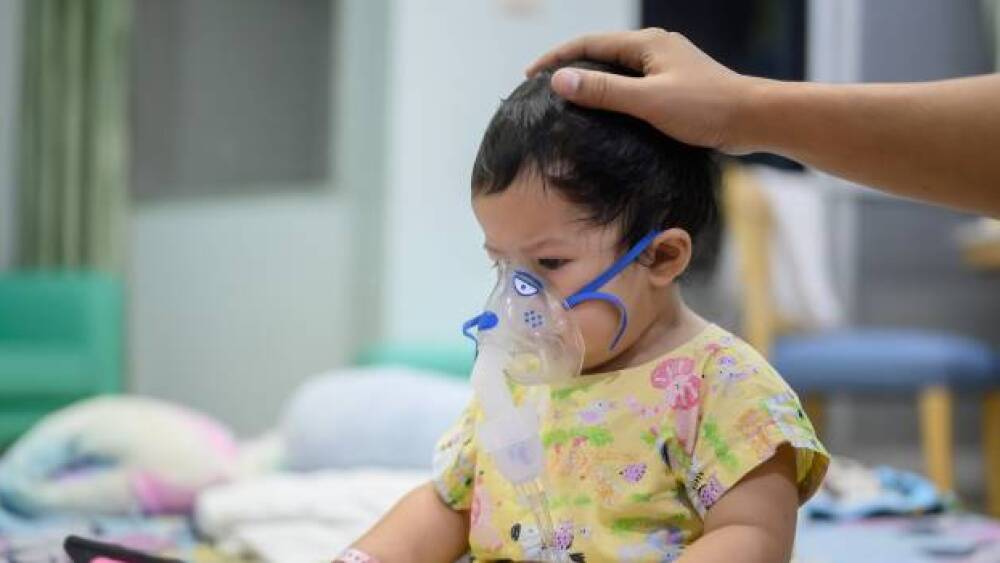
Between January 4, 2020 and October 30, 2021, COVID-19 accounted for 576 deaths among children 17 and younger, compared to 189 from influenza, according to the CDC.
Pfizer and BioNTech requested the FDA authorize booster shots for their COVID-19 vaccine for all adults 18 years and older, presenting data not available in September.
New studies come out regularly supporting the efficacy and safety of the COVID-19 vaccines. Here’s a look as well as other COVID-19 news.
Biohaven and Pfizer have partnered to market the Nurtec migraine medicine outside the U. S. once approved by global regulatory organizations. Here’s more about it.
JOBS
IN THE PRESS