Johnson & Johnson Family of Companies
NEWS
It was another busy week for both COVID-19-related clinical trial news as well as trial updates for other indications. Here’s a look.
Several biopharmaceutical companies have made headway as of late in the race to develop a vaccine for COVID-19, which is responsible for the current global pandemic.
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for October 13, 2020.
J&J did not disclose much information about the unexplained illness, stating, “We must respect this participant’s privacy. We’re also learning more about this participant’s illness, and it’s important to have all the facts before we share additional information.”
Biopharma companies wrapped up September and headed into October with plenty of clinical trial news. Here’s a look.
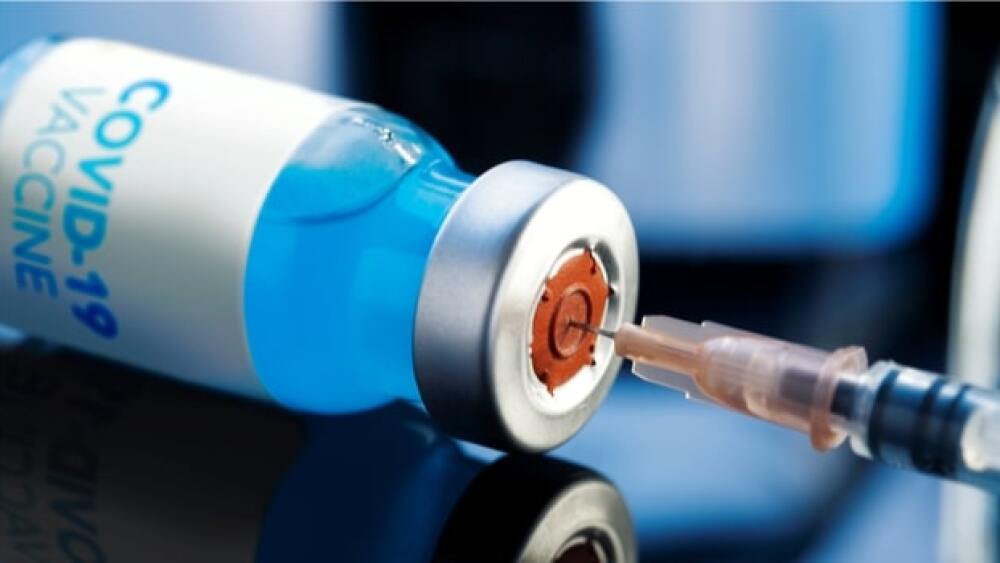
The company posted an interim analysis of its Phase I/IIa trial that showed a single dose of JNJ-78436735 induced a strong neutralizing antibody response in nearly all participants and was well-tolerated.
The European Society of Medical Oncology (ESMO) Virtual Congress 2020 was last weekend and the beginning of the week, which resulted in numerous clinical trial announcements. Here’s a look.
The J&J vaccine appears to require only a single dose. Several of the other candidate vaccines require two doses about 28 days apart.
Please check out the biopharma industry coronavirus (COVID-19) stories that are trending for September 23, 2020.
JOBS
IN THE PRESS