Telix Pharmaceuticals
NEWS
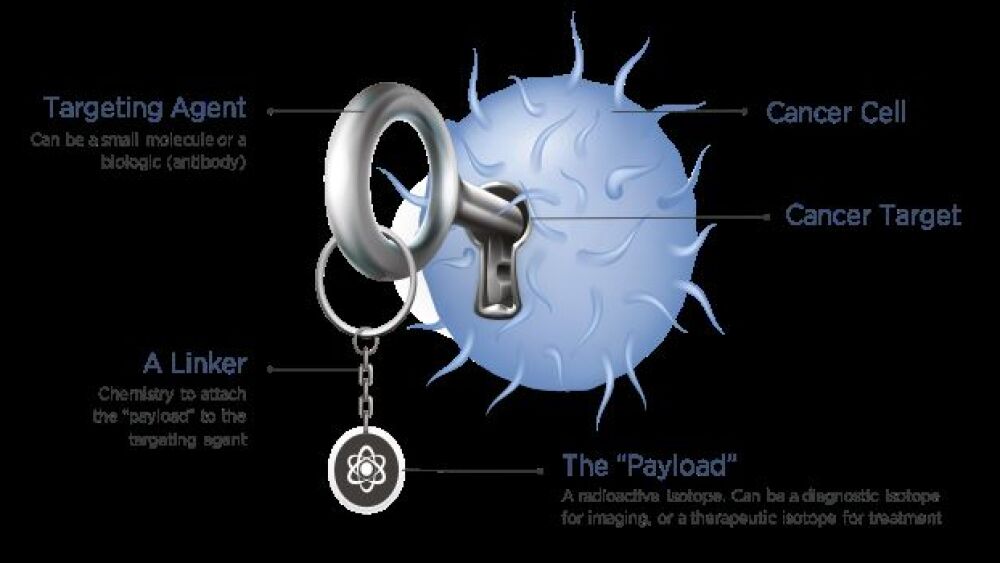
Pharmaceutical companies are combining antibodies with radioisotopes in a bid to more precisely deliver radiation to cancers and tumors.
Neuroscience-focused Rapport Therapeutics and radiopharma developer Telix Pharma announced their respective plans Friday for initial public offerings on the Nasdaq for undisclosed dollar amounts.
The week began with new partnerships formed in the life sciences industry as several firms entered into licensing deals and collaboration commitments. Here’s a look at the latest.
Biopharma and life sciences companies from across the globe provide updates on their businesses and pipelines.
Biopharma and life sciences organizations from across the globe provide updates on their businesses and pipelines.
It was yet another busy week for clinical trial news. Here’s a look.
Biopharma and life sciences companies from across the globe provide updates on their pipelines and business operations.
Modern radiation therapy is tightly targeted to dramatically reduce toxicity and side effects and often can be administered on an outpatient basis.
JOBS
IN THE PRESS