COVID-19
The data demonstrated that the drug, when given to the patients for more than seven days, offered a protection rate of 100%.
The FDA has stated that GSK and Vir’s COVID-19 treatment sotrovimab is no longer authorized to be used for the condition because of its low efficacy against Omicron sub-variant BA.2.
Moderna’s CEO Stéphane Bancel spoke at the Boston College Chief Executives Club on Monday and recapped the laundry list of vaccines and treatments the company plans to develop.
Stephane Bancel, the chief executive of Moderna, has likened the need for booster vaccine doses to the way Apple releases new smartphones every year.
Three studies supporting Merck and Ridgeback Biotherapeutics’ Lagevrio (molnupiravir) will be presented at the ECCMID 2022 in Lisbon from April 23-26.
Two companies in India have halted vaccine manufacturing, which could be a sign that the pandemic may be coming to an end. For that and more COVID-19 news, continue reading.
It was a particularly busy week for clinical trial announcements. Let’s take a look.
The landmark Human Challenge Programme deliberately exposed 36 young and healthy participants ages 18 to 30 with no immunity to the SARS-CoV-2 virus for 14 days.
Although the data suggests the vaccines are extremely effective and generally safe, analysis is still coming in about adverse events as well as effectiveness in specific patient populations.
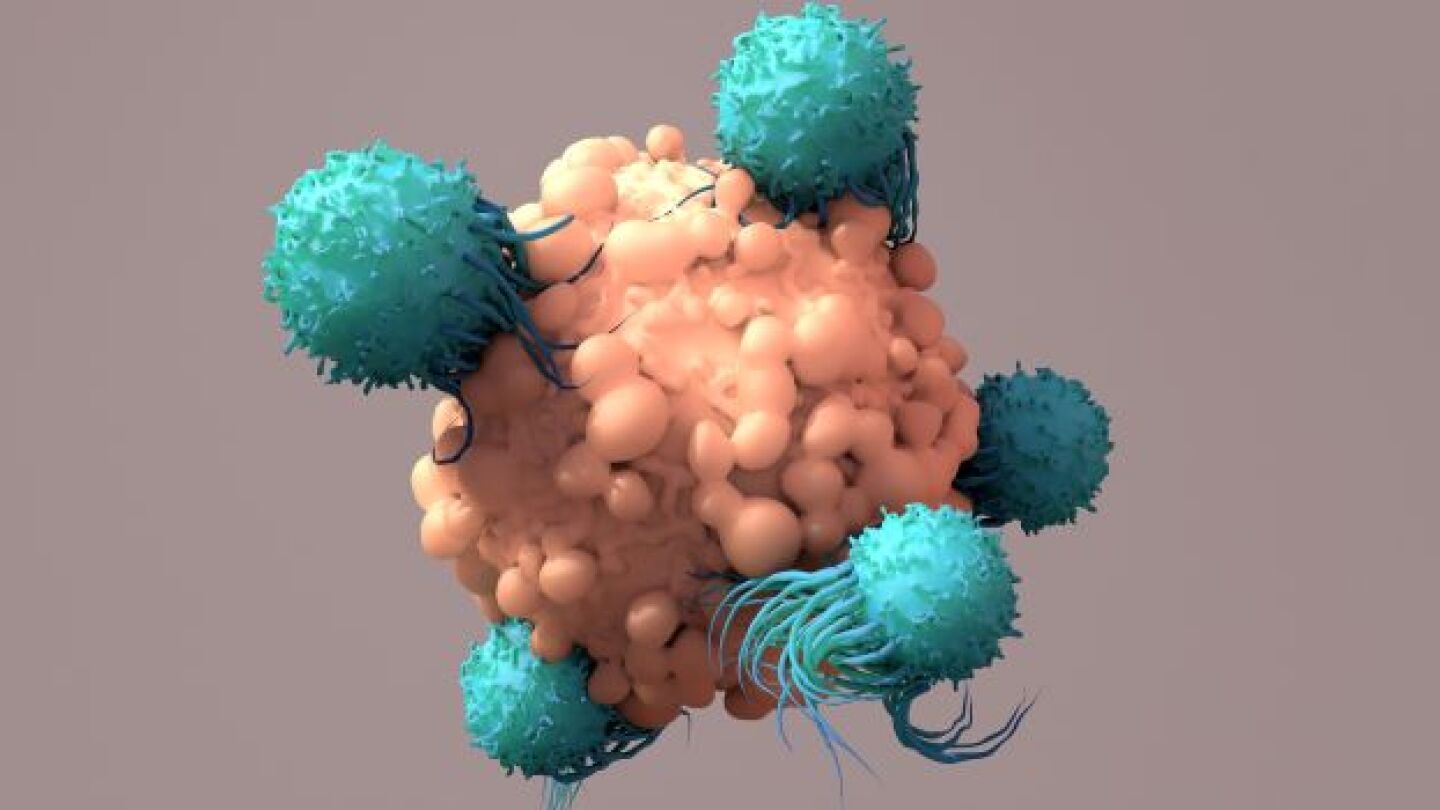
CAR-T cell therapy is still a time-consuming and expensive process. But researchers may have just solved the turnaround-time challenge for CAR-T.
PRESS RELEASES