All News
As Omicron continues to dominate globally, research is coming in every day on this highly contagious variant of SARS-CoV-2.
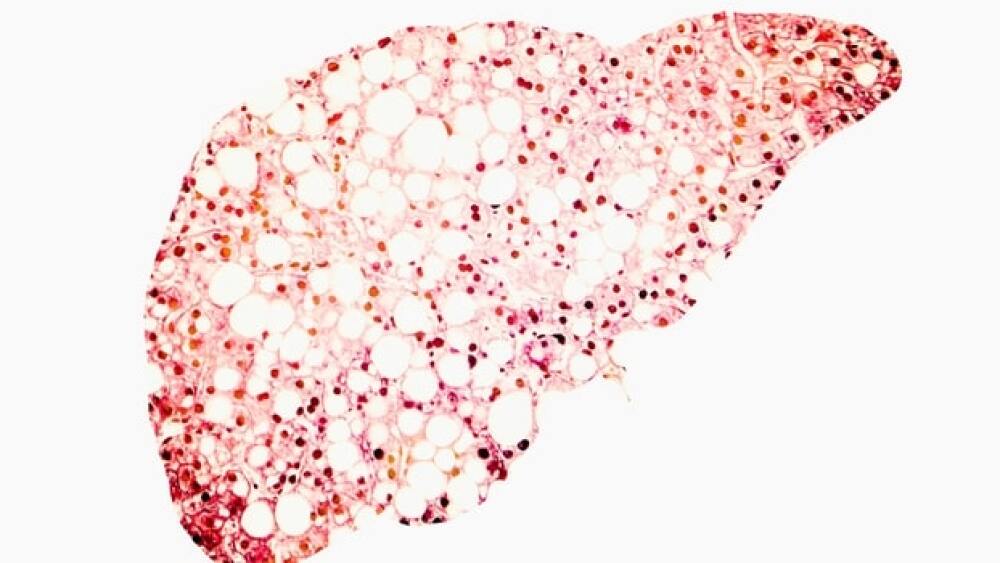
Sixty-three percent of patients met the primary endpoint of a 2-point or greater improvement in disease severity without worsening of fibrosis.
The FDA cited data demonstrating that both treatments are not likely to be potent against omicron and should not be authorized for use in any U.S. state, territory, and jurisdiction for the time being.
Dealing with a micromanager boss or coworker can be challenging. Read the full article on Biospace to find out how to deal with a micromanager at work.
The company submitted its application for AP-188 in early 2021, covering dosage details, formulation, and method of stroke rehabilitation.
BioSpace sat down with Niko Andre, M.D., Ph.D., global franchise head for hematology and immuno-oncology at AstraZeneca.
MaaT Pharma decided today to end the Phase Ib CIMON study for MaaT03, the company’s second drug candidate and first oral formulation in Phase 1b clinical trial, following positive interim engraftment data of the drug.
Oncopeptides has discontinued the marketing of Pepaxto drug in the US. But, in a recent turnaround, they decided to withdraw this decision of stopping the production.
The U.S. Food and Drug Administration issued a Complete Response Letter to Merck & Co. for its New Drug Application for gefapixant for refractory chronic cough or unexplained chronic cough.
FDA rejected OPKO’s once-weekly human growth hormone, somatrogon, which it co-developed with Pfizer for the treatment of growth hormone deficiency (GHD) in pediatric patients.
Suliman anticipates filing an Investigational New Drug application with the FDA for the PCD asset this year, and next year filing one for the cystic fibrosis asset.
TScan Therapeutics, Lyell, SQZ Biotech and others secured clearance from the U.S. Food and Drug Administration for their Investigational New Drug Application.
It’s a landmark no one expected or wanted — more than 70 million confirmed COVID-19 cases in the U.S. with 866,000 deaths. For that and more COVID-19 news, continue reading.
Last week was a relatively quiet week for clinical trial news, particularly around COVID-19. Here’s a look.
The U.S. Food and Drug Administration has approved SKYRIZI (risankizumab-rzaa), AbbVie’s proposed treatment for adults diagnosed with active psoriatic arthritis (PsA).
Gilead has announced they are pulling cancer drug Zydelig (idelalisib) off the market for certain types of cancer after failing to complete follow-up clinical trials to confirm efficacy and safety.
The San Diego-based immunology company is locating and developing molecules where the mechanism of action has either been proven or makes sense.
After decades of treating the big categories of heart disease – hypertension, for example – the ability to address the genetics of heart disease is within sight.
January has been a largely quiet month for PDUFA dates on the U.S. Food and Drug Administration (FDA)’s calendar. There were two dates for the entire month, and one of those has been moved back to April. Here’s a look.
Pazdur, along with the FDA’s R. Angelo de Claro and Gautam Mehta from the Center for Drug Evaluation and Research, penned an opinion piece published Thursday in JAMA Oncology.