All News
Retro Biosciences announced on Twitter that it is launching with $180 million in funding. Not much is known about the company, except that it indicates it is focused on the “cellular drives of aging.”
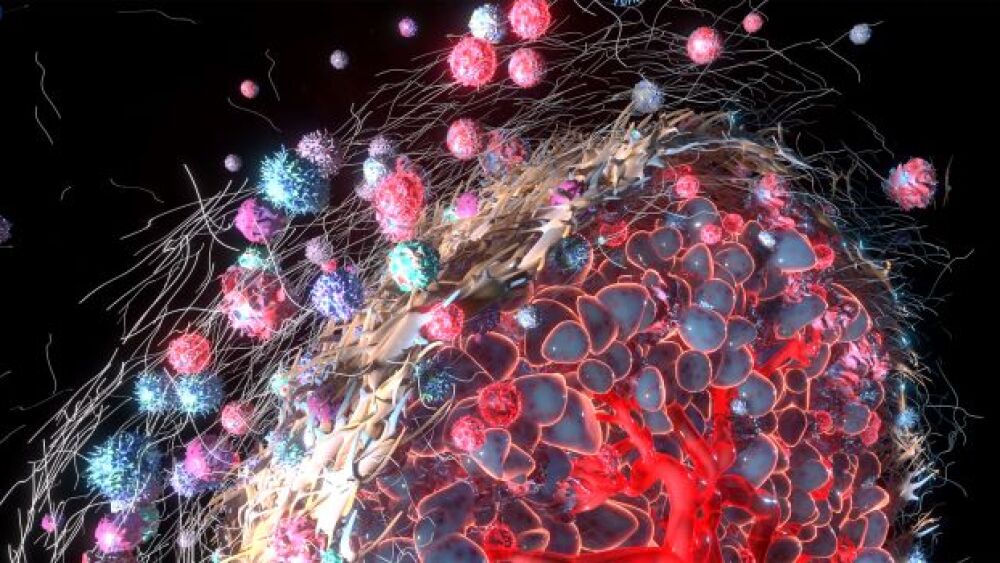
A team of scientists from Mount Sinai was able to uncover specific tumor microenvironment (TME) regulators using a novel functional genomics technology known as Perturb-map.
The data demonstrated that the drug, when given to the patients for more than seven days, offered a protection rate of 100%.
Biopharma and life sciences organizations from across the globe provide updates on their businesses and pipelines.
Evozyne inked a partnership with Takeda Pharmaceutical to develop next-generation gene therapies for up to four rare disease targets. Continue reading for that and more collaboration news from this week.
The AMR Action Fund financed two of this week’s money moves. Other moves ranged from medical devices to wearable sensor data companies. For that and more, continue reading.
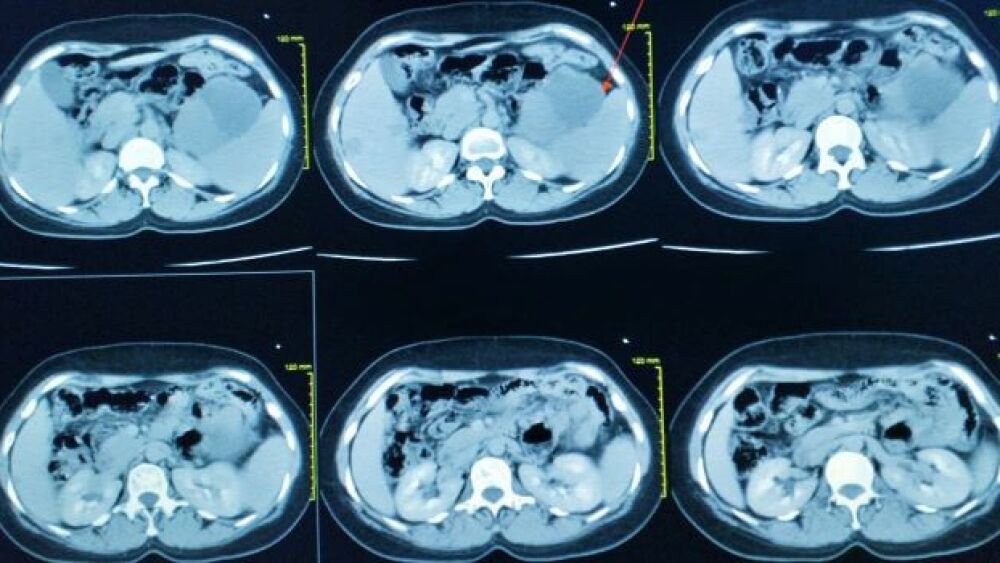
Citius Pharmaceuticals has shared positive topline data from its Phase III clinical trial of I/Ontak. The company plans to file a biologics license application with the FDA later this year.
Galen Robotics will further benefit from Robert Langer’s extensive experience in biotech startups and hands-on laboratory work.
Here’s a look at the eight most profitable biopharma companies around the world. Profitability, in this case, refers to net income, as opposed to total revenue or sales.
With the accelerated approval from the U.S. Food and Drug Administration, Vijoice becomes the first treatment approved in the United States for PROS
The FDA has stated that GSK and Vir’s COVID-19 treatment sotrovimab is no longer authorized to be used for the condition because of its low efficacy against Omicron sub-variant BA.2.
Boehringer Ingelheim made a splash on Tuesday, announcing its intention to pump €25 billion ($27 billion) into its R&D pipeline over the next five years.
Noted Harvard researcher Doug Melton is heading to Vertex Pharmaceuticals to head up investigations into potential transformative treatments for type 1 diabetes.
The U.S. Food and Drug Administration (FDA) has approved BioXcel Therapeutics’ Igalmi, a drug designed to reduce agitation episodes in bipolar disorder and schizophrenia patients.
Athira Pharma is circling the wagons and urging shareholders to reject investor Richard Kayne’s calls for a management change.
Researchers at the Perelman School of Medicine at the University of Pennsylvania have discovered a novel type of cell buried in healthy human lung tissue.
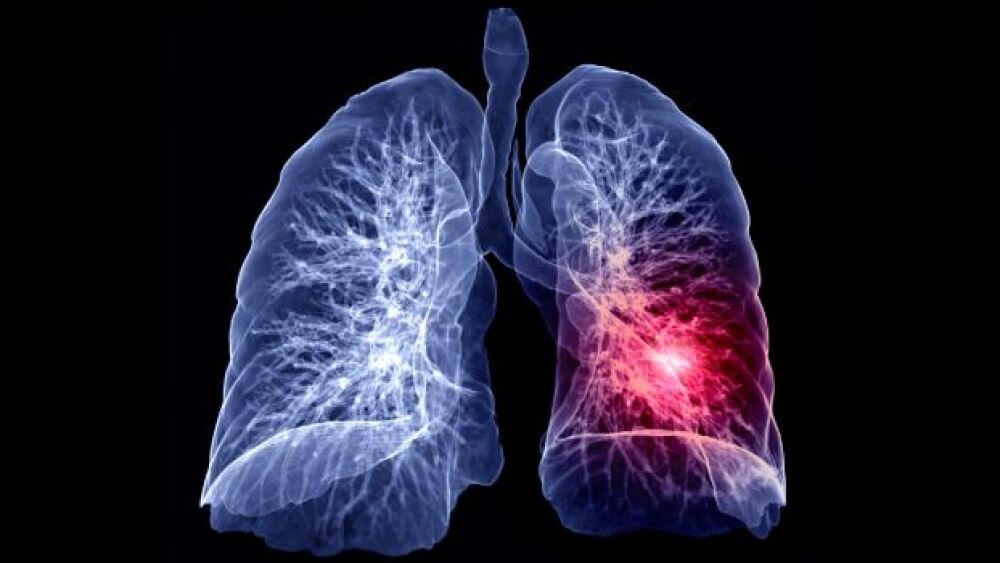
Speakers at the 2022 Virtual Growth Conference’s session on pancreatic cancer acknowledged the exceptional challenges while extolling advances in immune-based therapies and combination approaches.
Requesting disability accommodations is vital for your success in the workplace. You can find out everything about how to ask for accommodations in our guide.
BMS partnered with digital oncology company GRYT Health to convene a series of “courageous conversations” between patients, practitioners, caregivers, clinical trial teams and public health leaders.
Celeris Therapeutics and Boehringer Ingelheim (BI) have announced they are entering into a collaboration to develop next-generation targeted protein degraders.